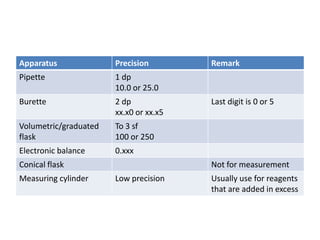
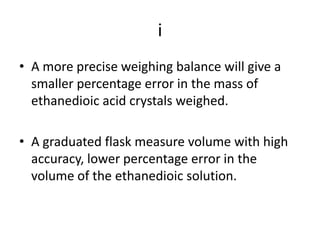
This document provides the experimental procedure for a titration experiment involving sodium hydroxide (NaOH) and ethanedioic acid. Key steps include accurately weighing ethanedioic acid crystals, preparing standard solutions in graduated flasks, using a pipette and burette to measure volumes, transferring all solid washings to the graduated flask, using an indicator to determine the endpoint, and only adding 1-2 drops of indicator. The procedure aims to increase precision and accuracy of measurements to obtain reliable concentration values for NaOH.



















![FactsNaOH solution being basic can absorb CO2 from the air.Amount of NaOH present in the solution is lesser than expected.[NaOH] < expected](https://image.slidesharecdn.com/practical4-100326034248-phpapp02/85/Practical-4-21-320.jpg)





![iiMore precise apparatus is used to measure the volume of the solution decreases the percentage error of volume measured.Using a pipette to 1 dp, ensures that an accurate volume of 10 cm3 acid is obtained. An accurate amount of acid is present in the conical flask for titration.Using a burette to 2dp ensures that the volume of NaOH used is more accurately determined, and therefore a more accurate [NaoH]](https://image.slidesharecdn.com/practical4-100326034248-phpapp02/85/Practical-4-30-320.jpg)

![iiiEnsure all the ethanedioic acid that was not transferred into the graduated flask and present in the washings was transferred.This ensures that the amount of ethanedioic acid present in graduated flask = amount of ethanedioic acid weighed.It will give rise to an accurate volume of NaOH used and therefore, accurate [NaOH]](https://image.slidesharecdn.com/practical4-100326034248-phpapp02/85/Practical-4-32-320.jpg)


![vIndicators are also weak acids/bases that can also take part in the titrationExcessive use will cause significant reactions to occur with the indicatorsTitration values will be higher/lower than expected[NaOH] determined will be lower/higher than actual](https://image.slidesharecdn.com/practical4-100326034248-phpapp02/85/Practical-4-36-320.jpg)