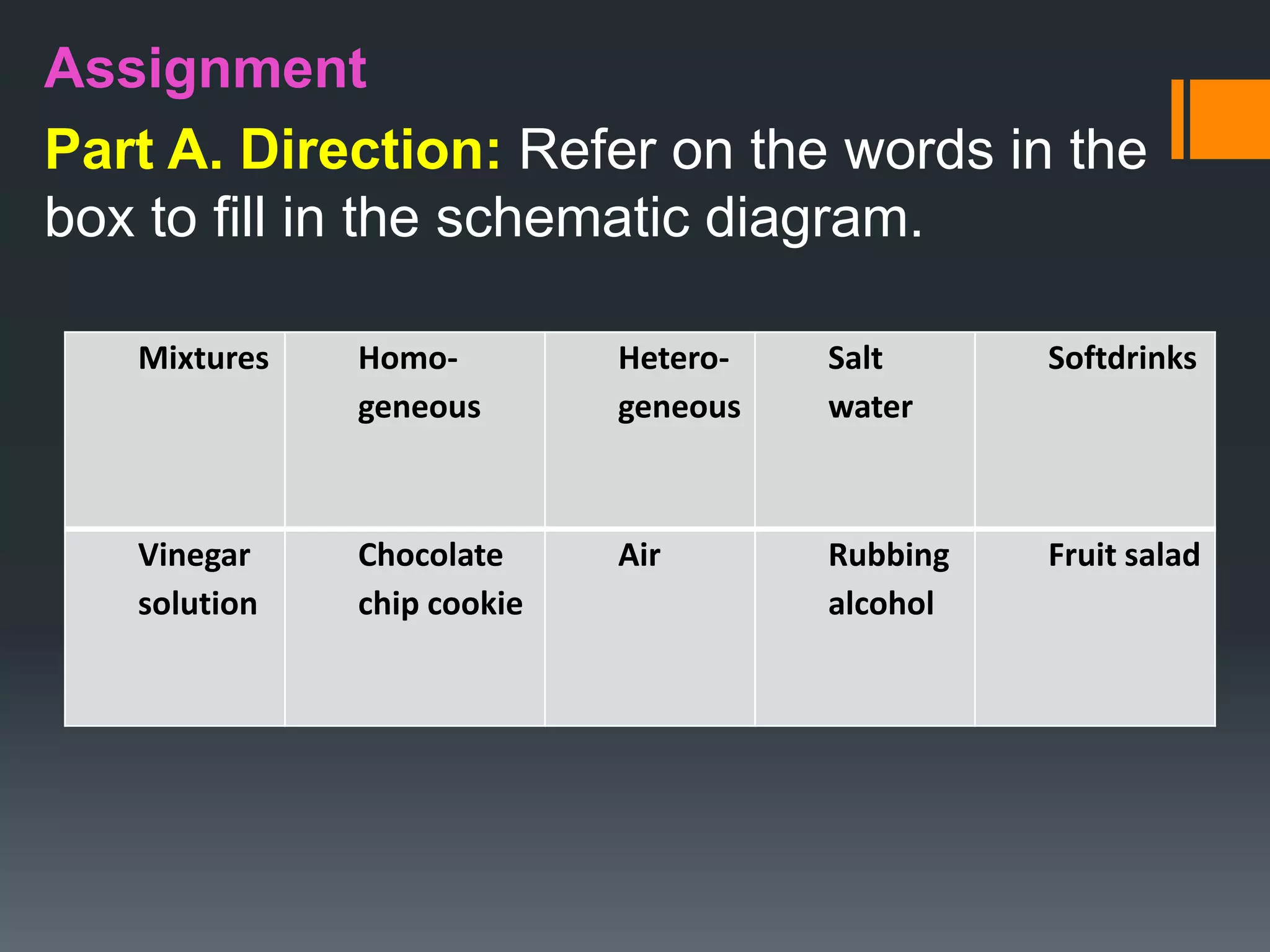
This document provides information about mixtures. It begins by stating the objectives of describing mixtures based on their components, uniformity, and composition. Key terms related to mixtures are defined, including heterogeneous mixture, homogeneous mixture, solute, solution, and solvent. Examples of homogeneous and heterogeneous mixtures are given through classroom activities where students observe mixtures like marble in water and vinegar in water. It is explained that heterogeneous mixtures have distinguishable components, while homogeneous mixtures appear uniform. Various types of solutions are discussed as examples of homogeneous mixtures. Students are assessed on their understanding through true/false questions and a diagram activity distinguishing mixture types.