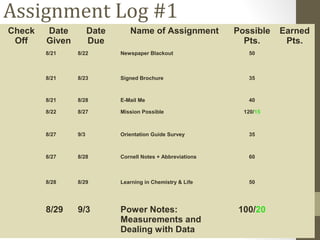
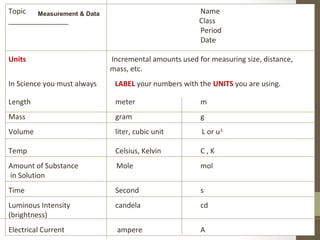
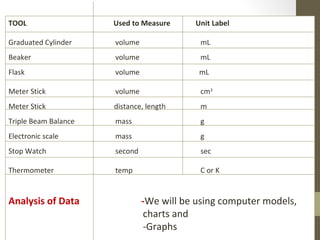
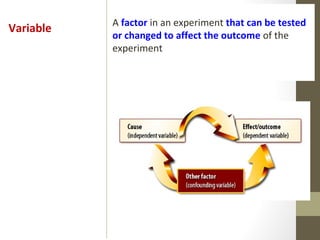
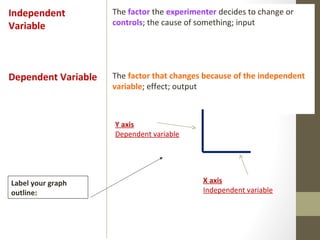
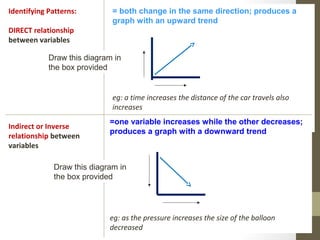
This document provides instructions and assignments for a chemistry class. It includes a checklist of assignments with due dates, instructions for an in-class quick write assessment, notes on extra credit opportunities, corrections to a crossword puzzle, and directions for completing power notes on measurements and dealing with data. Students are asked to have materials ready, log into an online system, and work on completing their power notes during class. Homework includes finishing any past due assignments and completing the power notes by the following Tuesday.