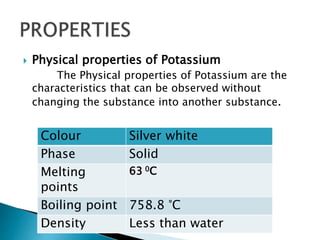
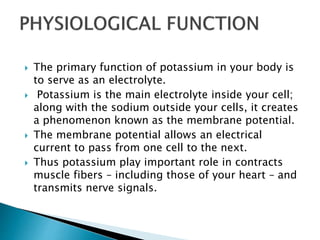
Potassium was first isolated in 1807 by Sir Humphry Davy through electrolysis of molten potassium hydroxide. It is a soft, silvery-white metal that burns with a lilac color flame. Potassium plays an important role in the human body as a major electrolyte to help muscles and nerves function properly and is found in foods like beans, potatoes, bananas, and yogurt. Deficiency can cause weakness, numbness, nausea and irregular heartbeat while adequate intake through diet is important for overall health and organ function.