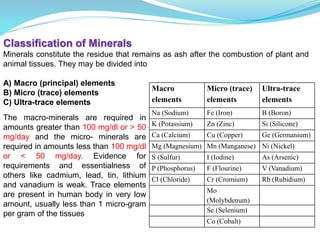
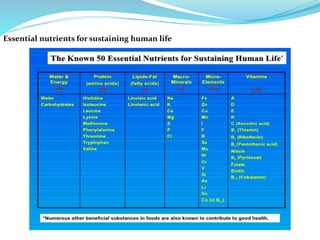
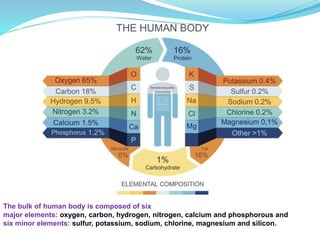
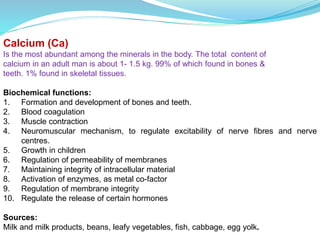
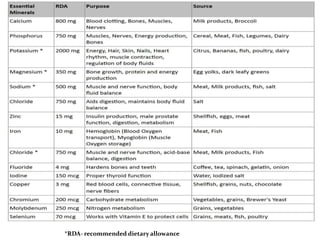
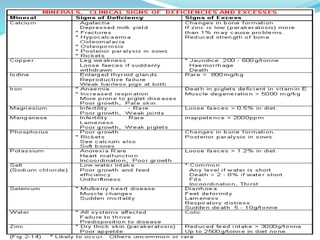
Minerals are essential inorganic substances necessary for various body functions, including bone building, fluid regulation, and energy conversion. They are categorized into macro, micro, and ultra-trace elements, with specific dietary needs and health impacts associated with each. Essential nutrition is grounded in minerals derived from both plant and animal sources, playing vital roles in physiological processes and overall health.