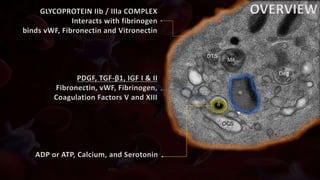
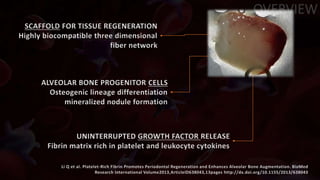
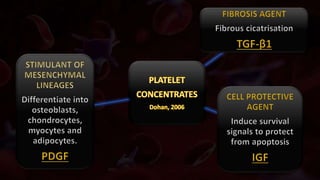
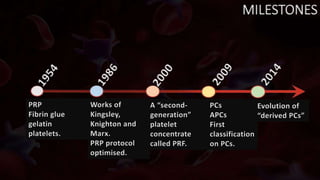
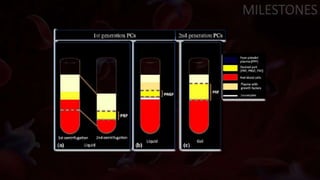
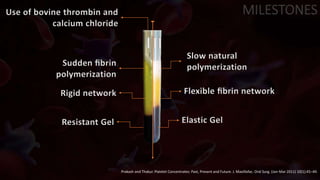
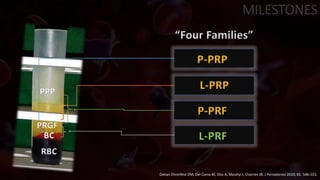
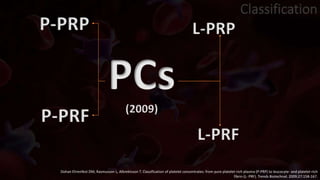
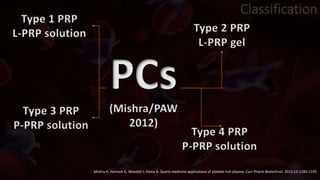
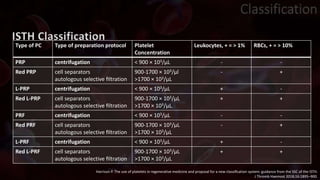
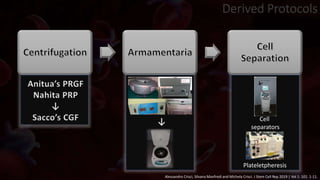
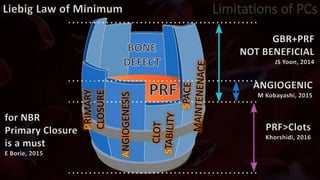
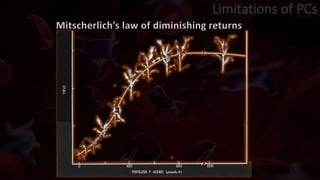
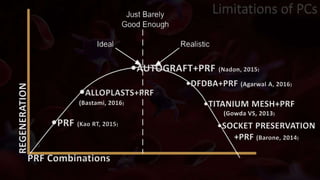
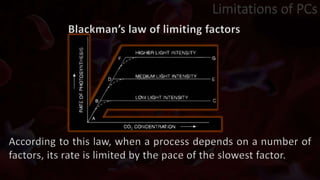
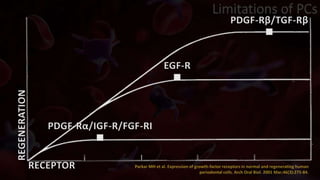
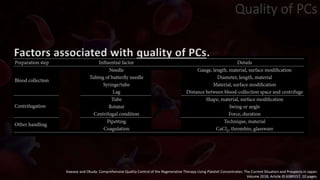
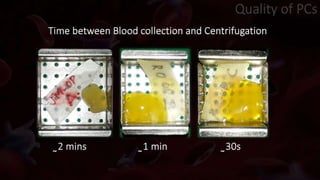
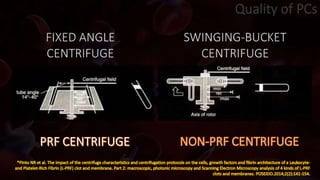
The document provides an update on platelet concentrates, discussing their interactions with various proteins and factors crucial for tissue regeneration, including detailed classifications and milestones in their development. It outlines the evolution of protocols and the issues surrounding the quality and management of platelet concentrates. The paper highlights both the advancements in clinical applications and the limitations that need addressing for optimal use in regenerative therapies.