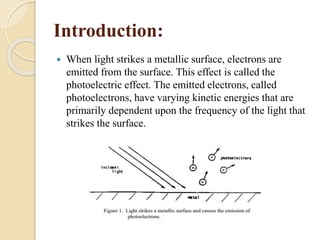
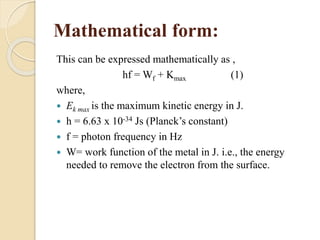
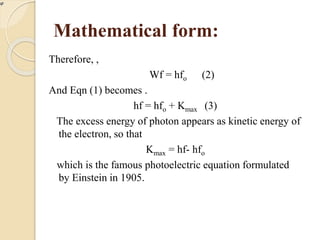
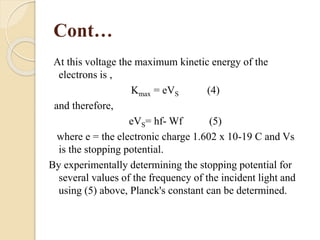
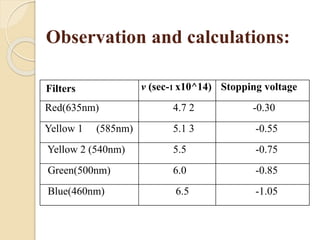
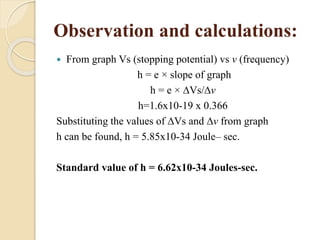
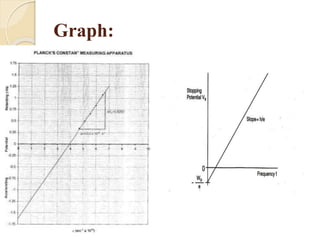
This document describes an experiment to measure Planck's constant using the photoelectric effect. The experiment involves measuring the stopping voltage of electrons emitted from a photocell when different wavelength photons strike the photocell. A graph of frequency versus stopping voltage is plotted and the slope is used to calculate Planck's constant. The calculated value of 5.85x10-34 Joule– sec is close to the accepted value of 6.62x10-34 Joules-sec, demonstrating the experiment's success in measuring Planck's constant.