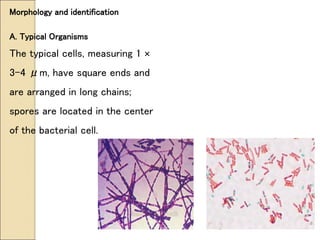
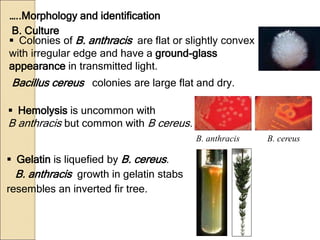
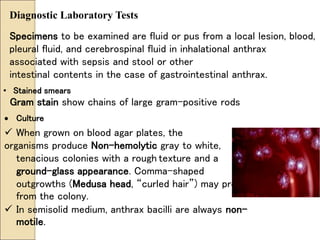
The document discusses the history, epidemiology, clinical presentation, diagnosis, and treatment of plague. Some key points:
- Plague caused 20 million deaths in Europe during the 14th century Black Death pandemic. It has also been studied as a biological weapon.
- Caused by the bacterium Yersinia pestis, plague typically occurs in 10-15 cases per year in the southwest US through flea bites. Pneumonic plague can spread from person to person.
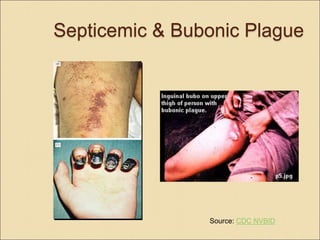
- Clinical forms include bubonic, septicemic, and pneumonic plague. Pneumonic plague has the highest mortality if not treated quickly with antibiotics.