1. Explosives are reactive substances that contain potential energy that can be released suddenly as heat, light, sound and pressure.
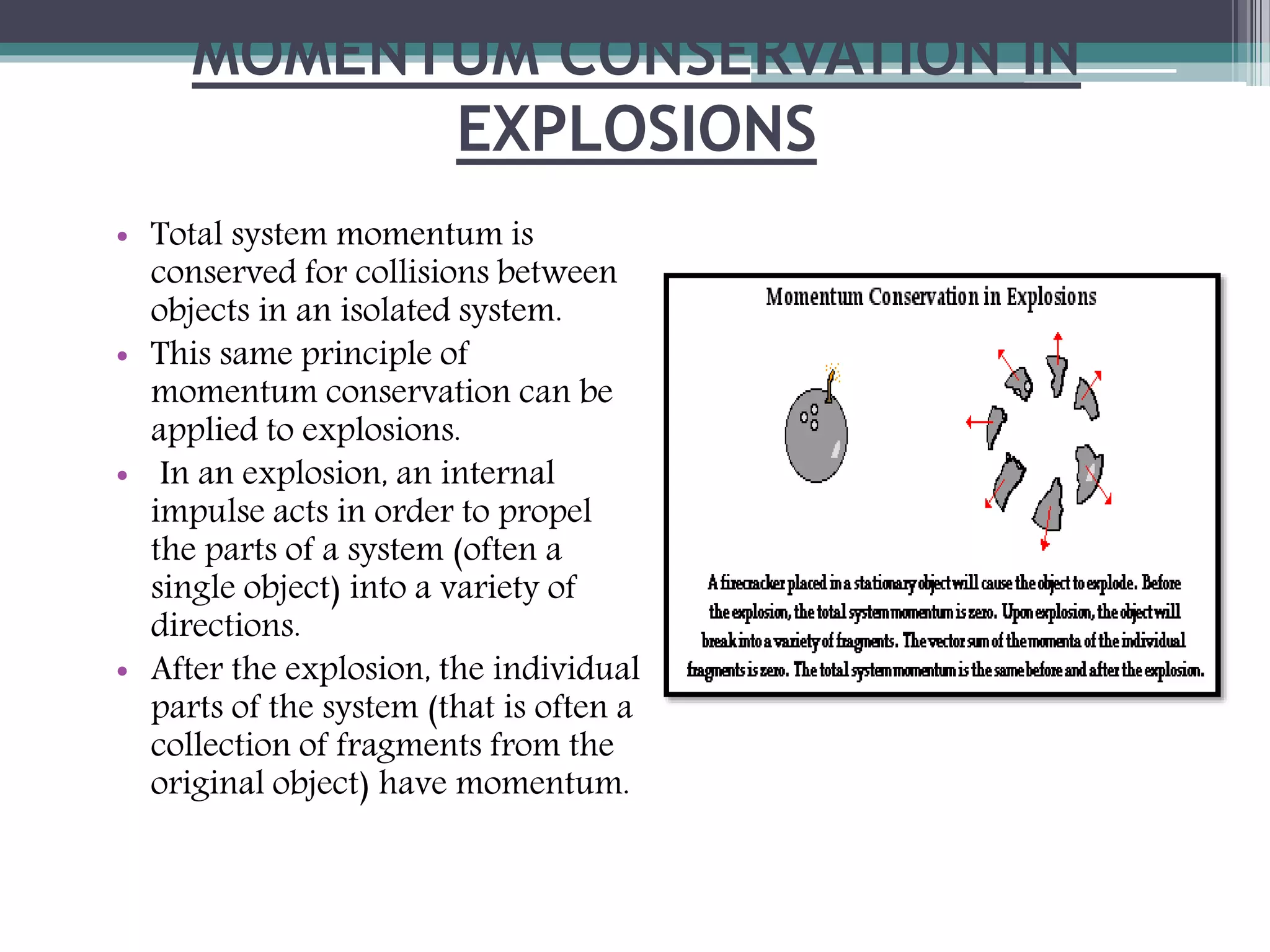
2. Chemical explosions are the most common and result from rapid oxidation reactions that produce large amounts of hot gas. Nuclear explosions involve fission or fusion reactions that release tremendous energy.
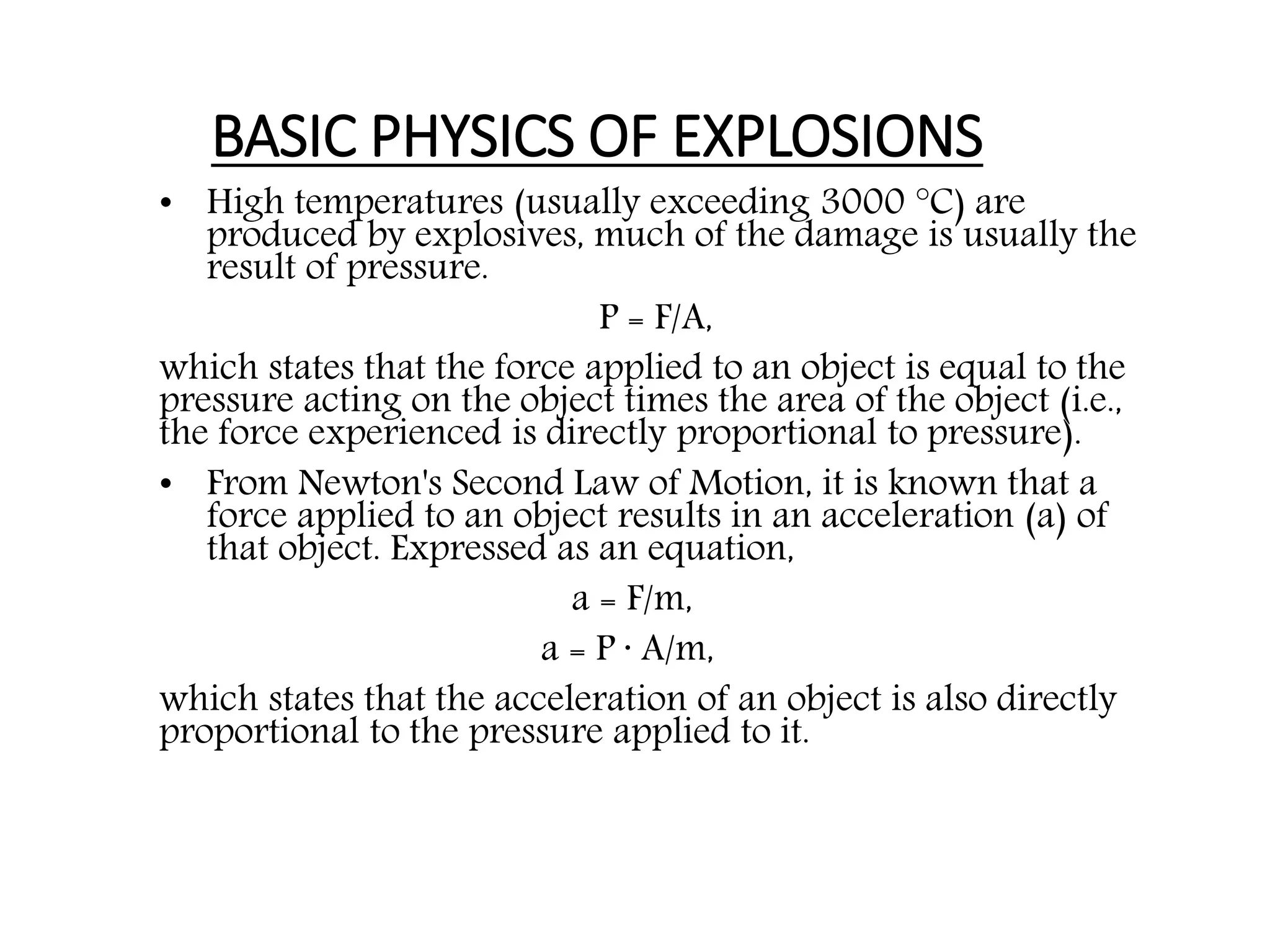
3. The key factors that cause an explosion are the reaction speed, which must be instantaneous, and a large increase in volume from reactants to products, such as from solids or liquids converting to gases. A tight container enhances the explosion effects.