Embed presentation
Download to read offline

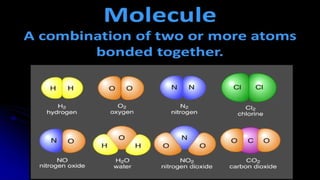
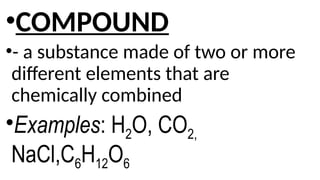
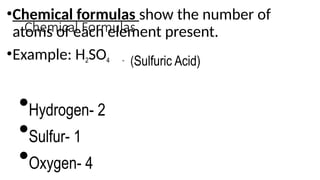

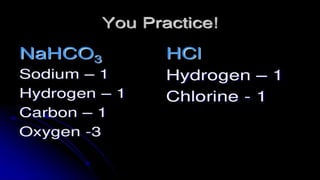





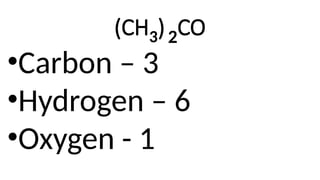

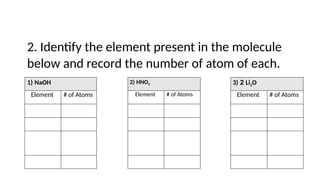
The document provides an overview of chemical compounds, including definitions and examples such as H2O and NaCl. It explains chemical formulas and how to count atoms using subscripts and parentheses. Additionally, it includes exercises for identifying elements and their atom counts in given molecules.














