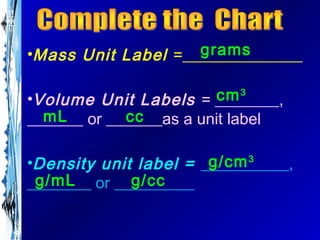
The document provides information about compounds and their formulas. It explains that subscripts in formulas indicate the number of atoms present of each element in a compound. Examples are given of common compounds like calcium carbonate (CaCO3), magnesium hydroxide (Mg(OH)2), and trinitrotoluene explosive (C7H5(NO2)3). The main elements that make up living organisms are identified as carbon, phosphorus, hydrogen, oxygen, nitrogen and sulfur. The document also lists common unit labels for mass, volume, and density.