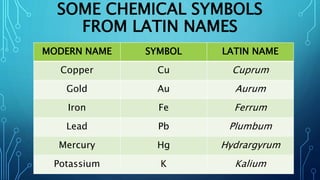
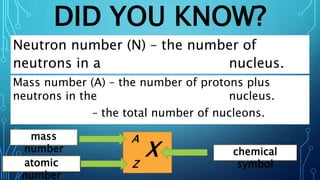
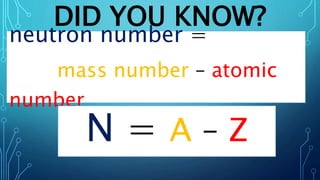
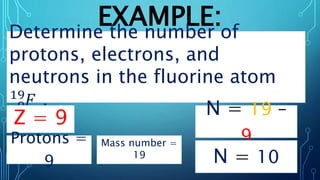
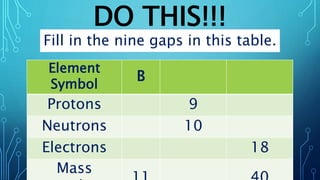
Physical science is the study of inorganic matter and includes four main branches: astronomy, physics, chemistry, and the Earth sciences. The nucleus of an atom, made up of protons and neutrons, contributes over 99.97% of an atom's mass. Atoms are identified by their atomic number, which is the number of protons, and isotopes of the same element have different numbers of neutrons but the same number of protons.