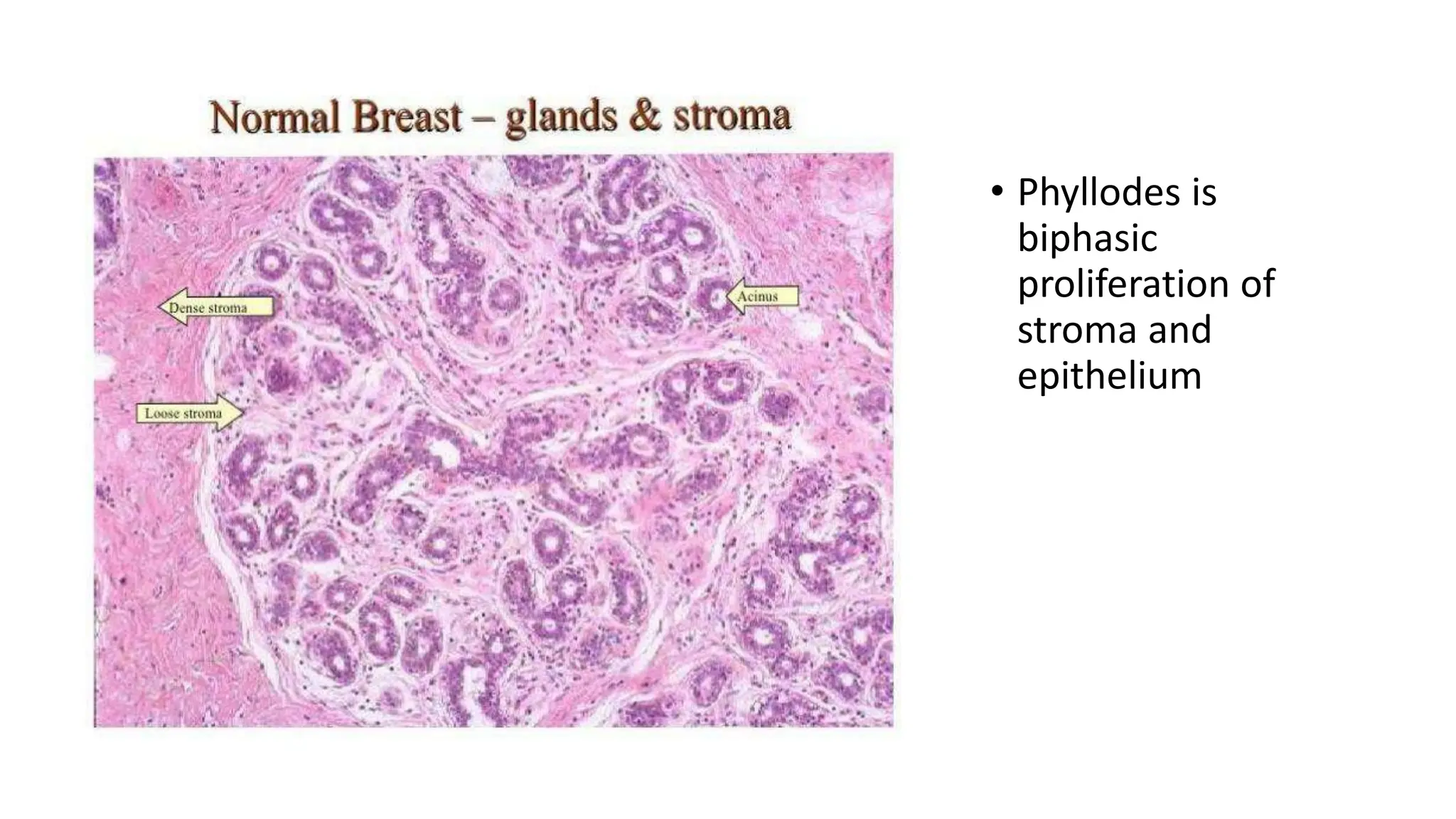
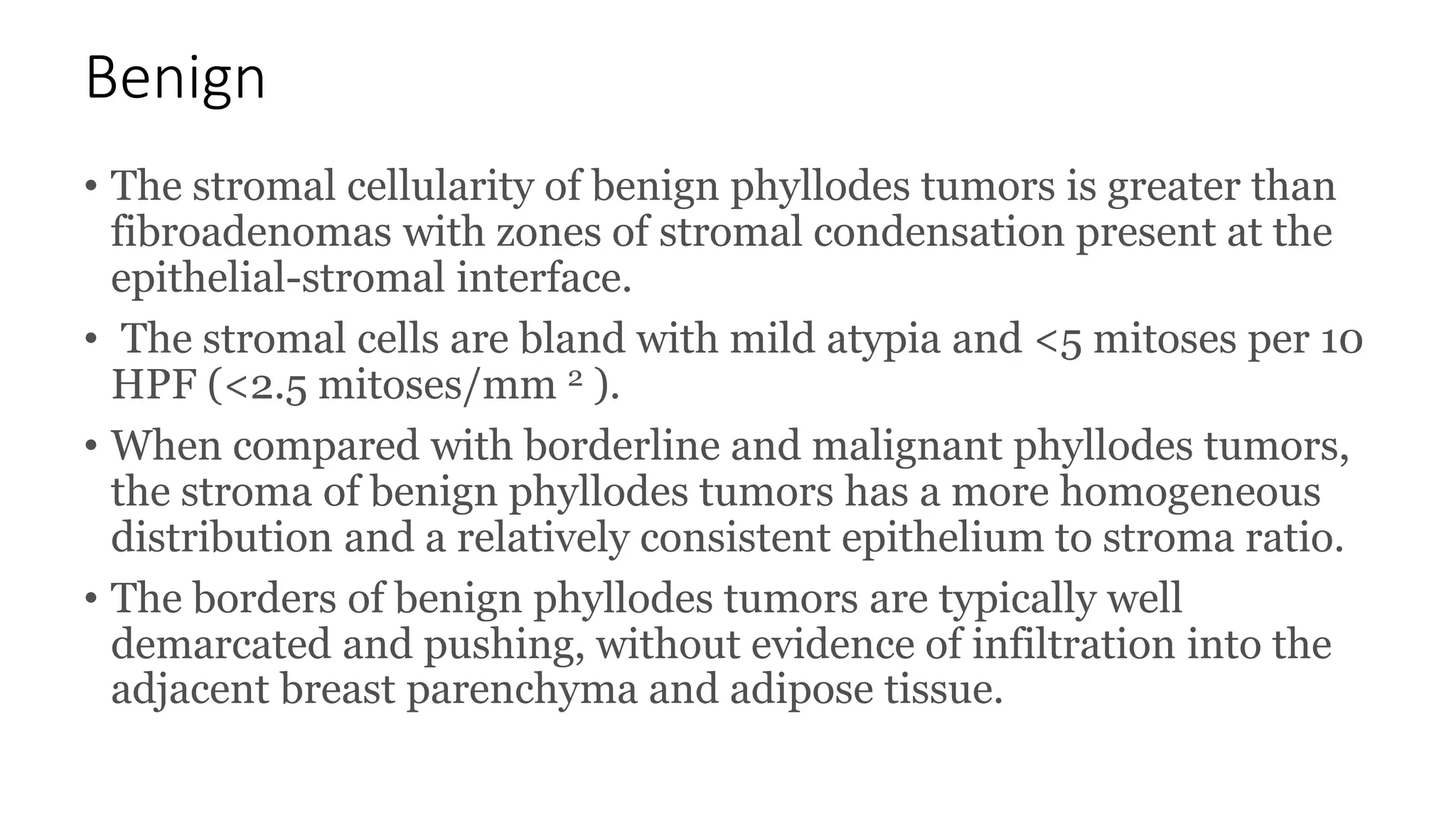
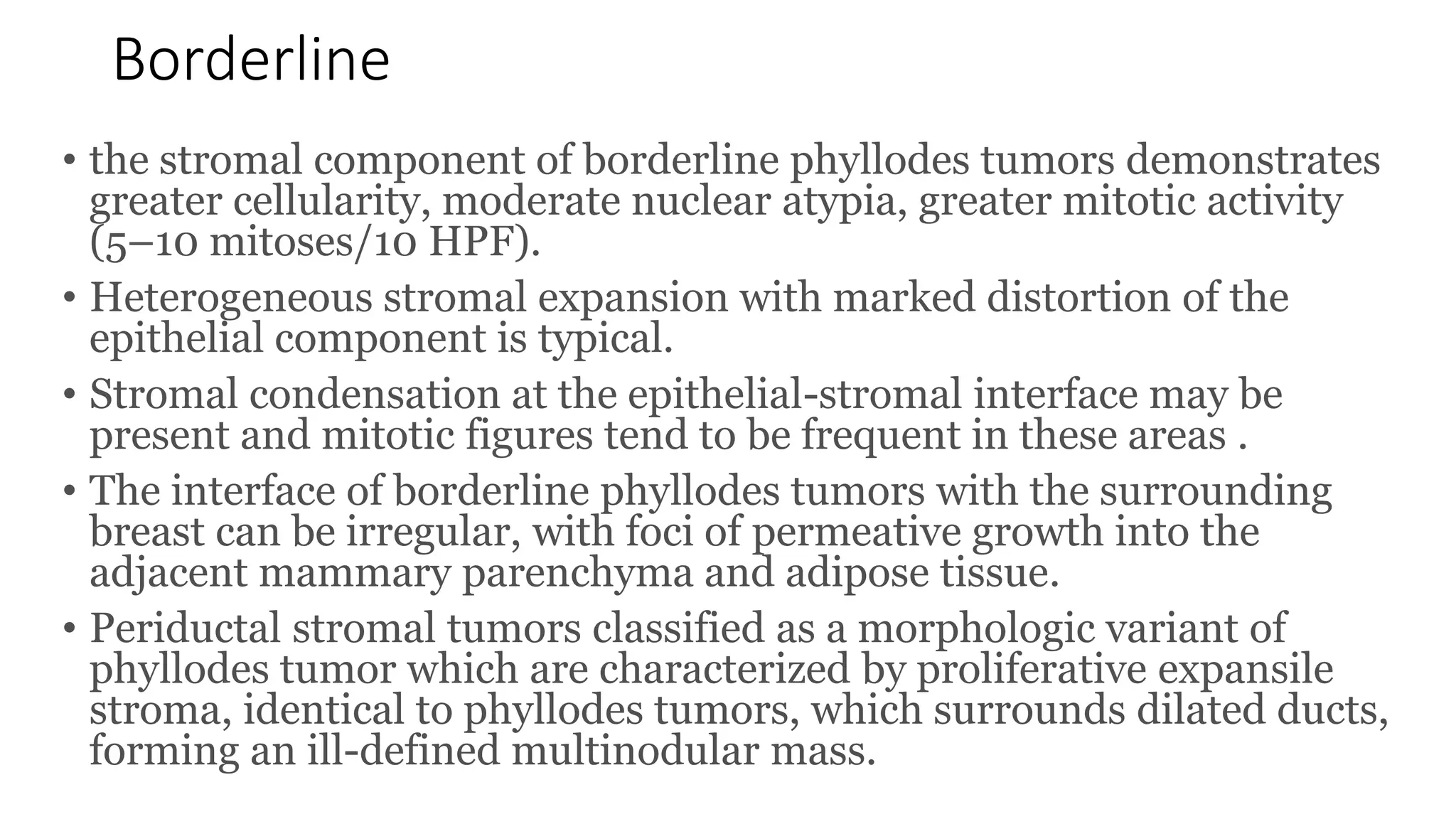
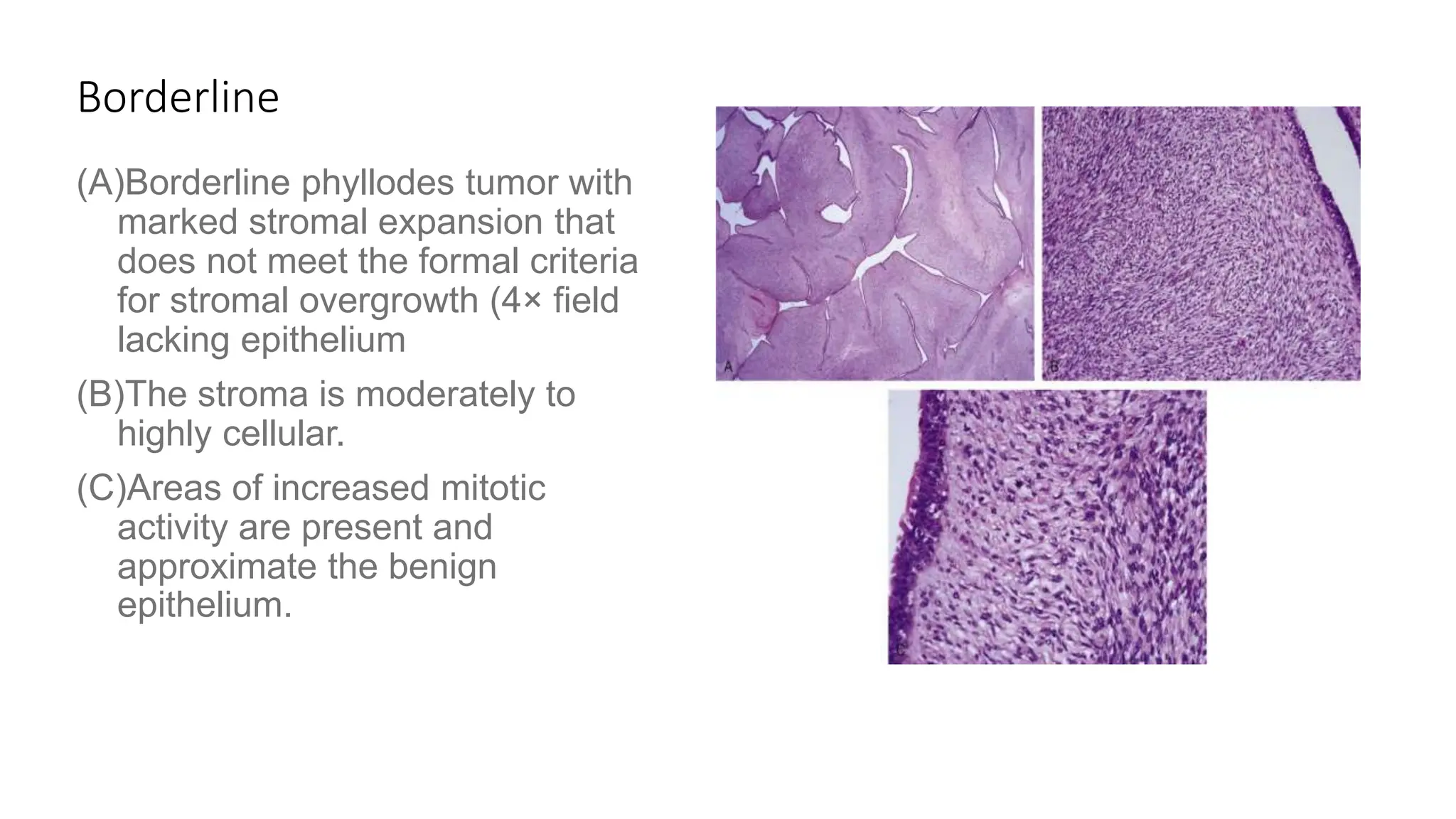
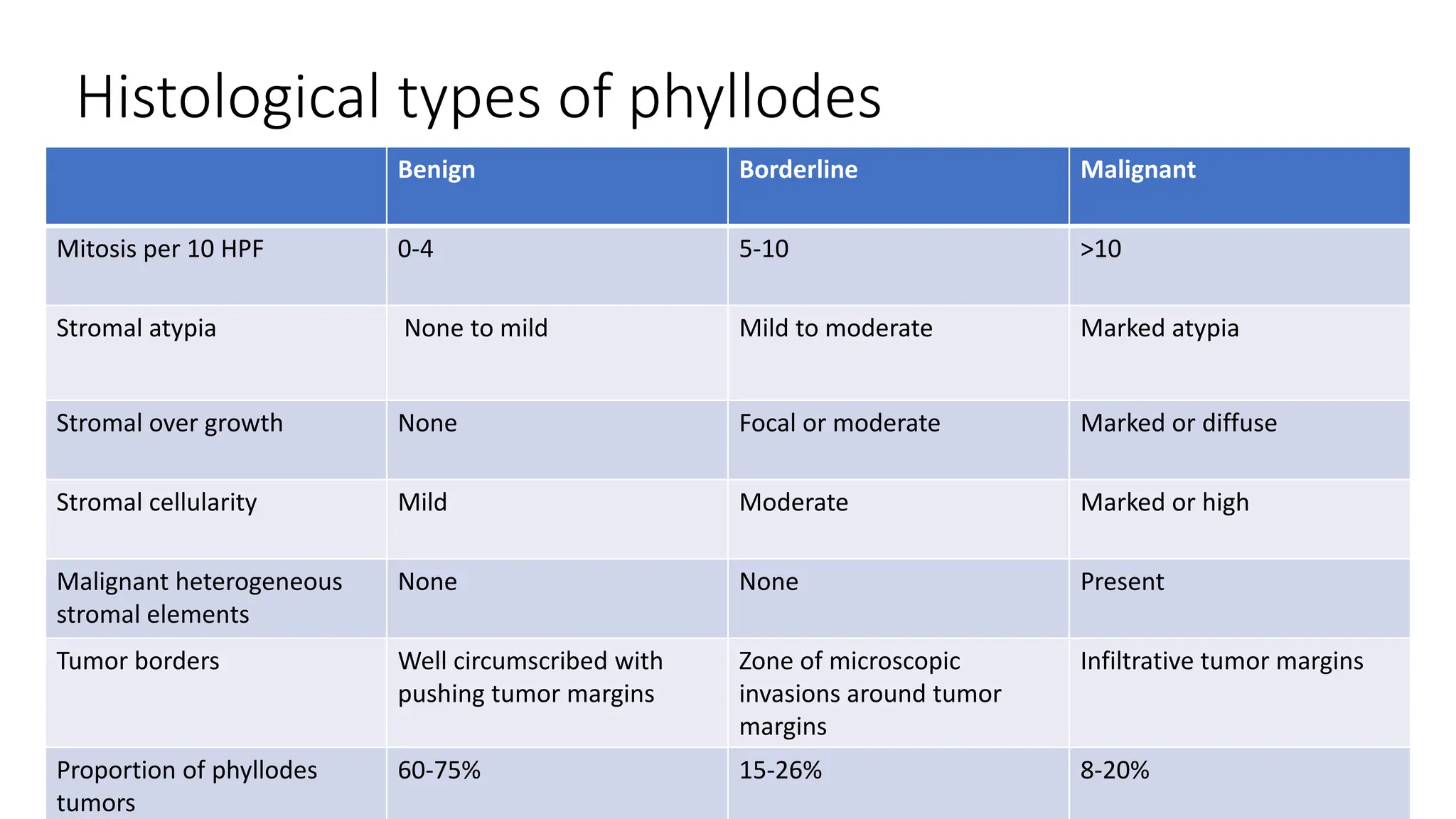
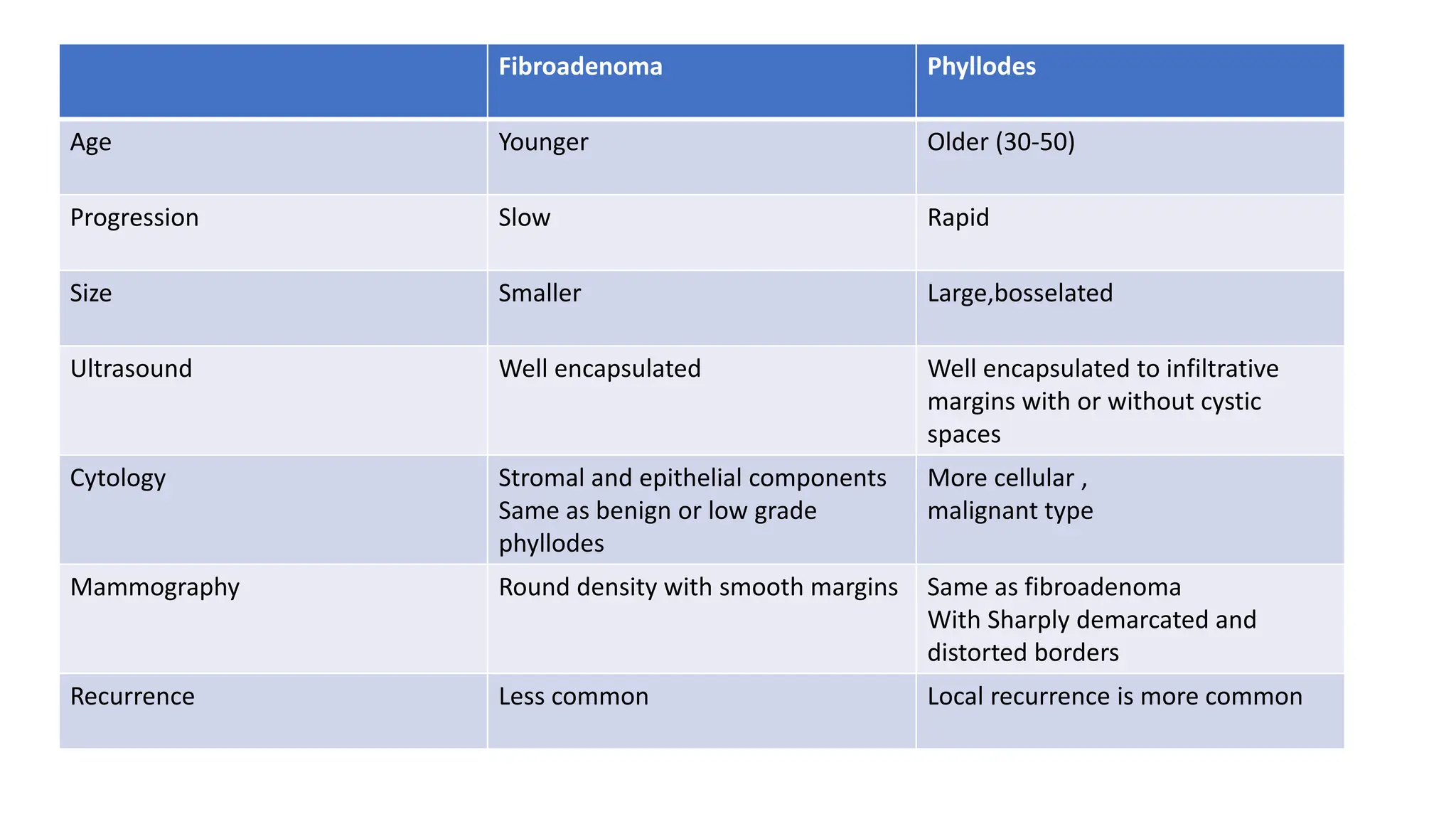
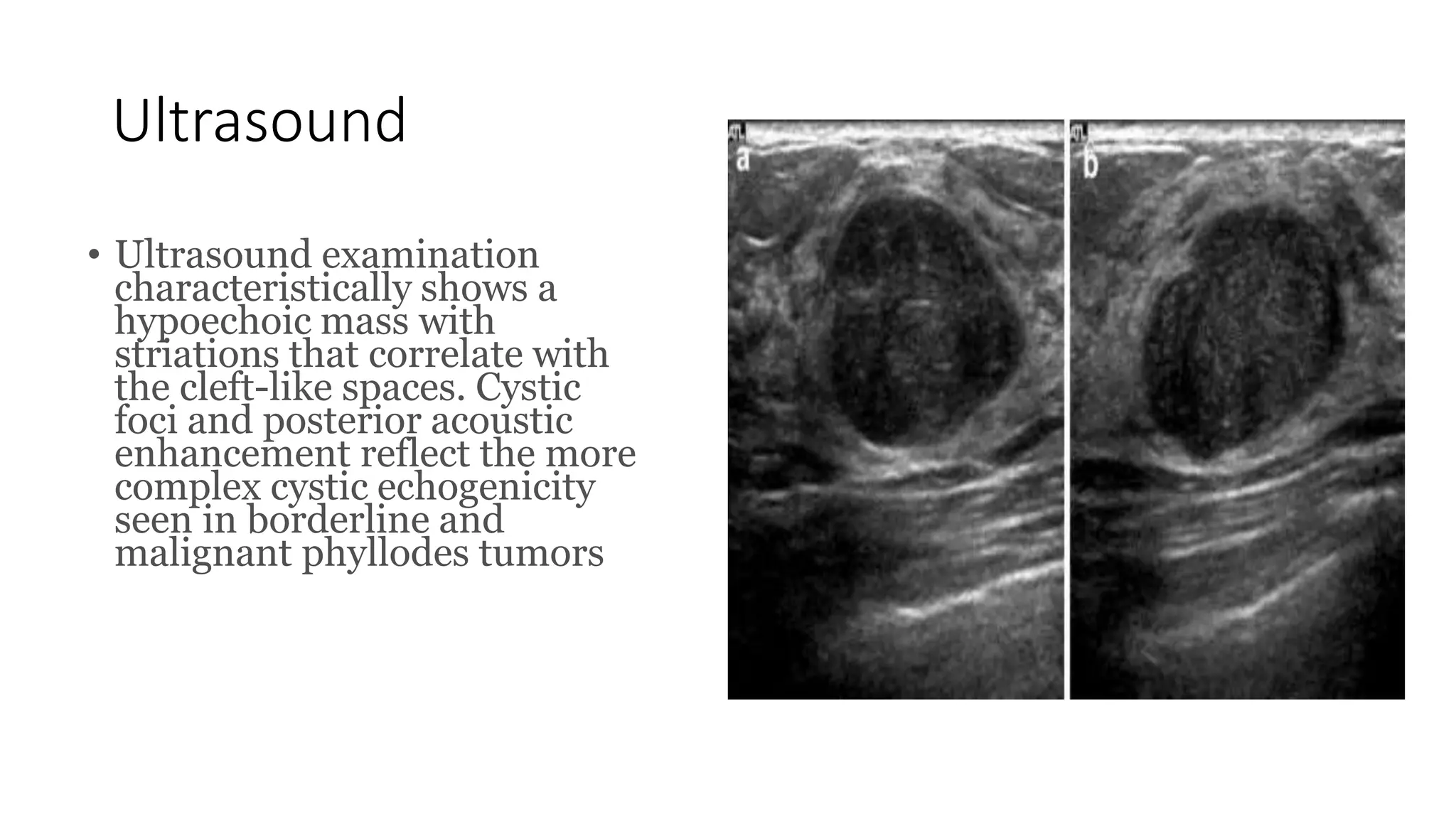
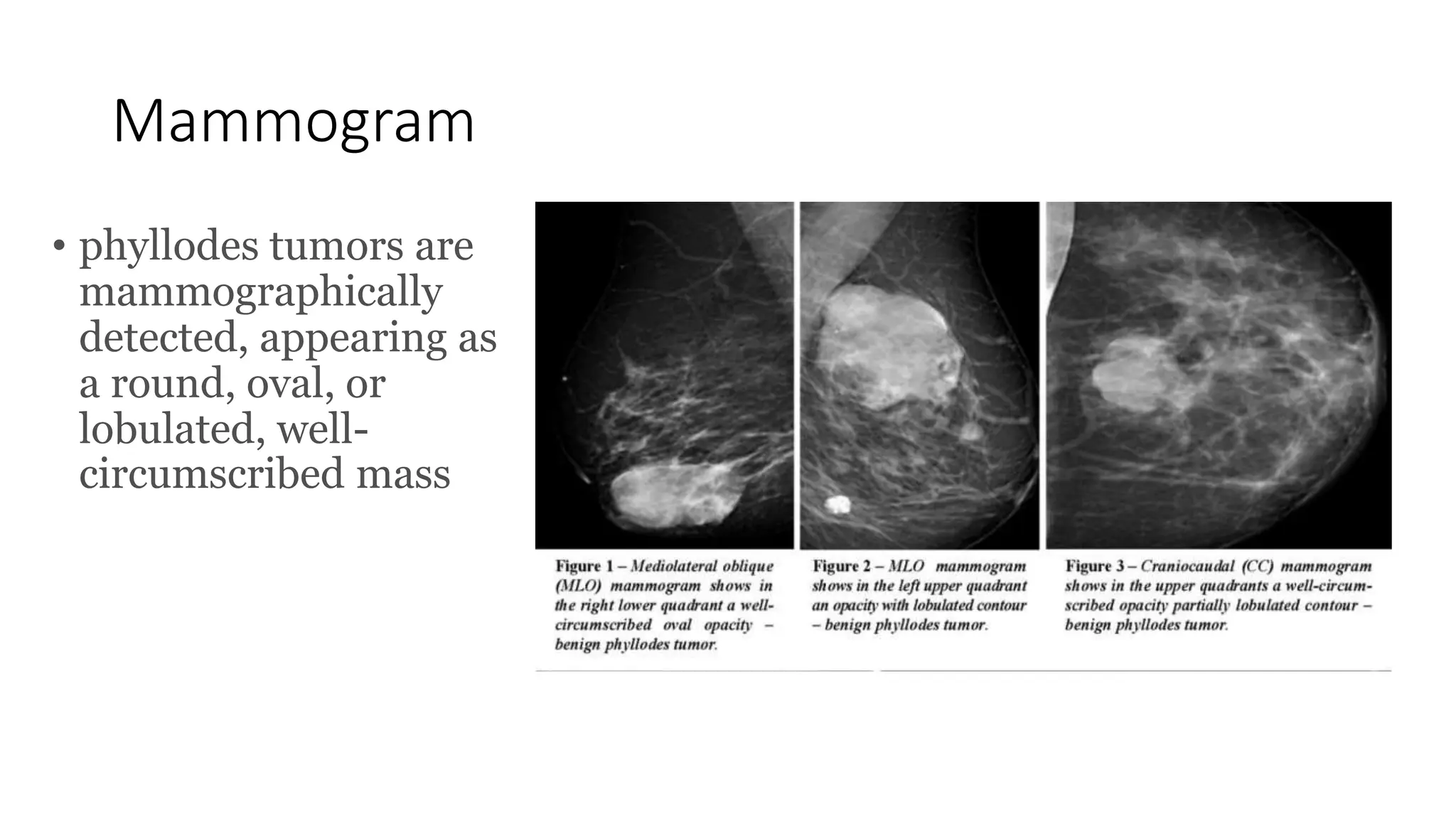
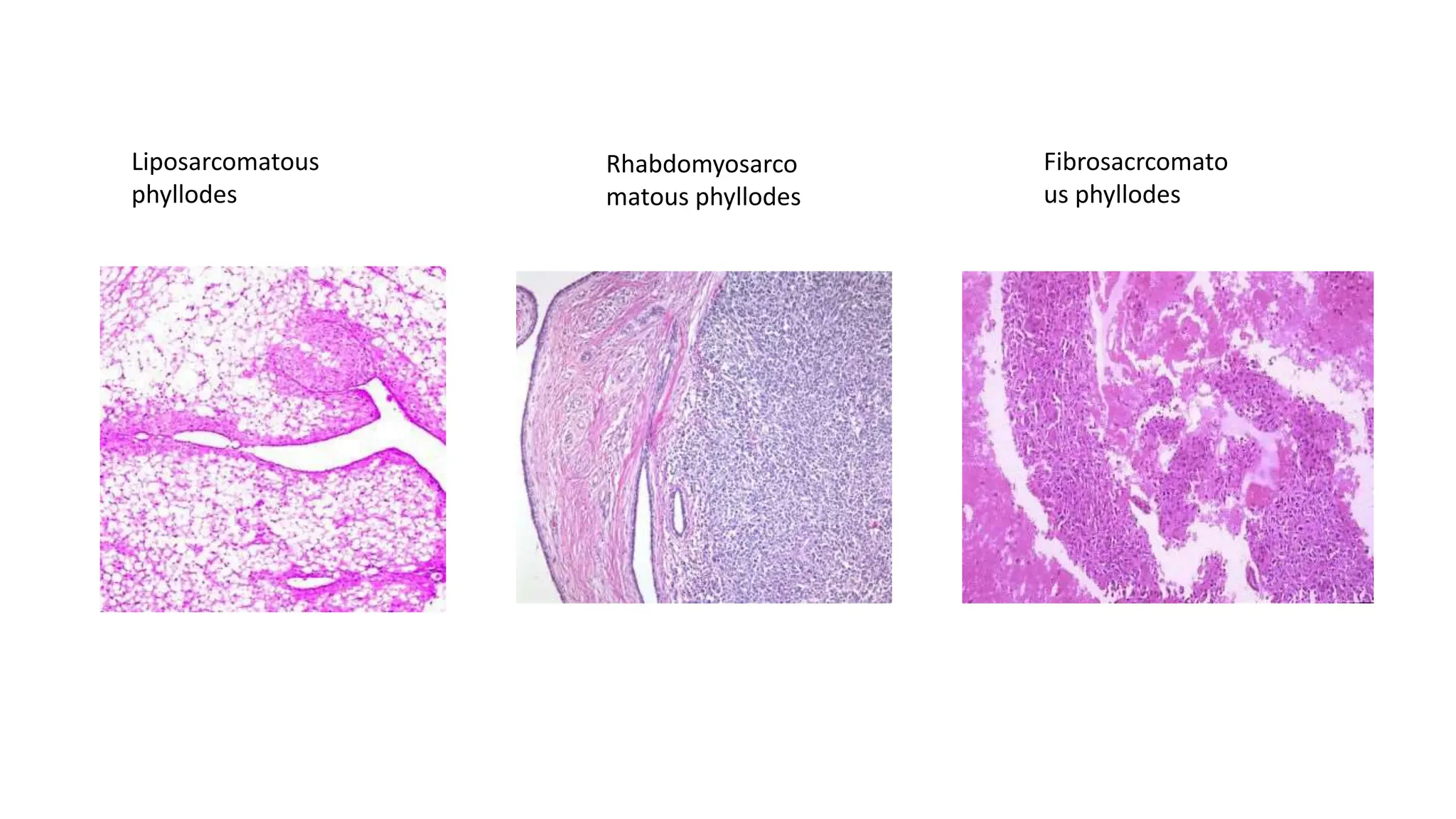
1) Phyllodes tumor is a rare fibroepithelial tumor of the breast that is classified as benign, borderline, or malignant based on histological features.
2) Surgical excision with adequate margins is the primary treatment, with mastectomy reserved for large or recurrent tumors.
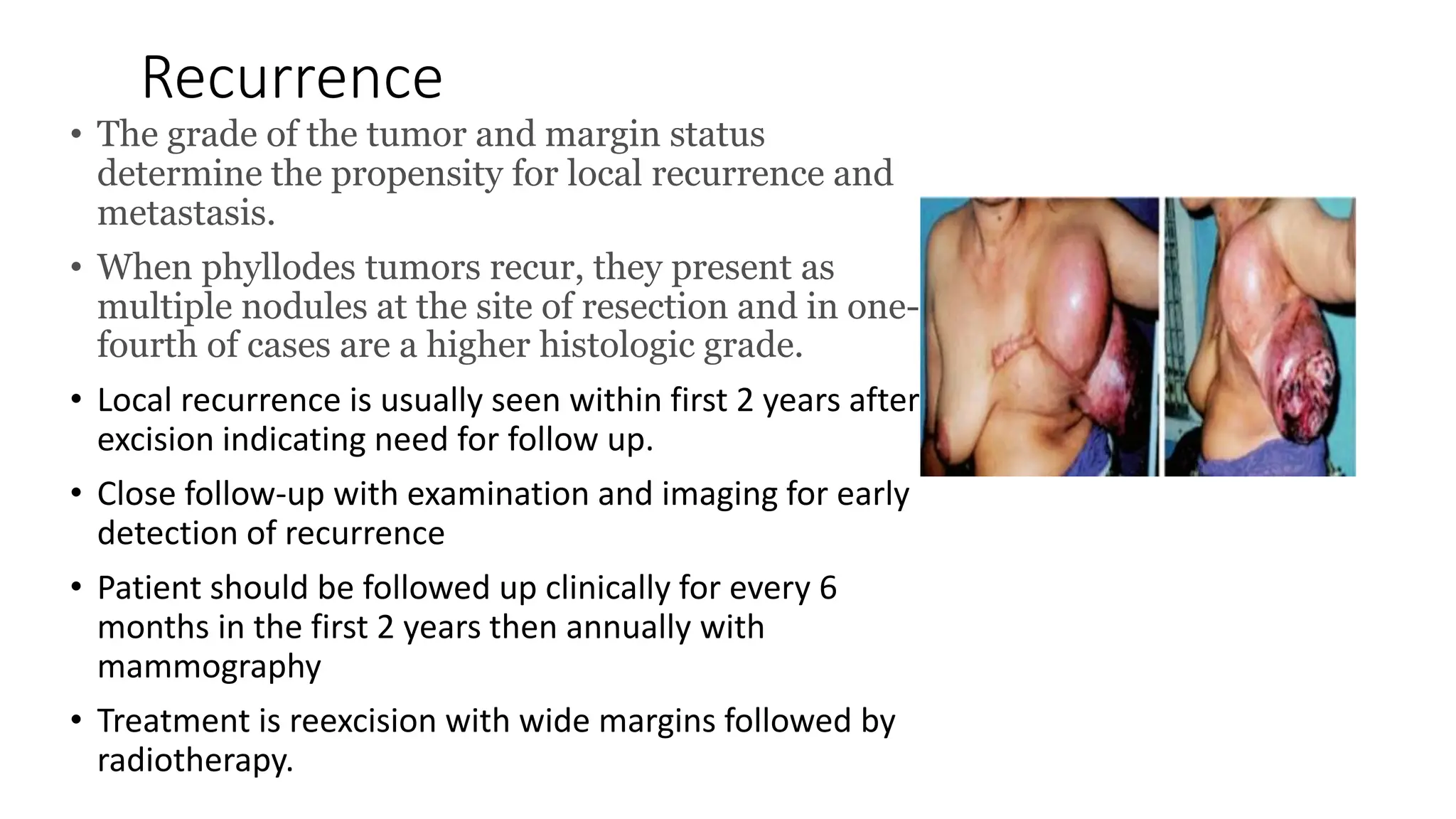
3) Prognosis depends on histological grade and margin status, with local recurrence rates of 10-30% depending on classification and a rare risk of distant metastasis for malignant types. Close follow-up is important to monitor for recurrence.