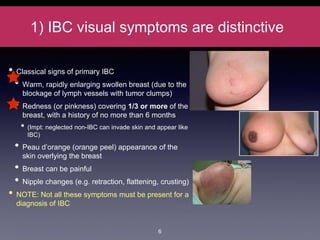
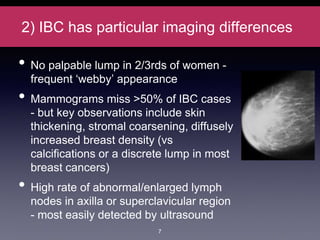
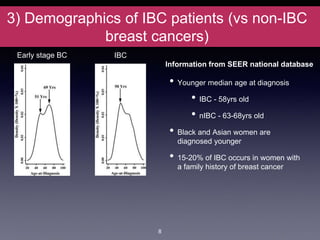
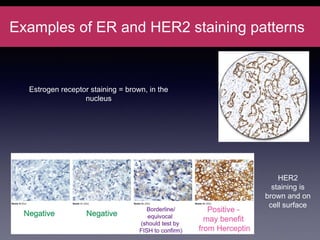
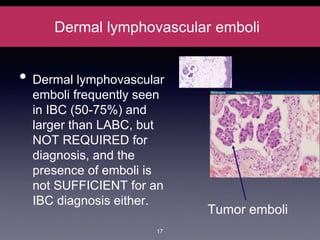
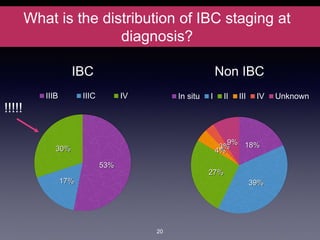
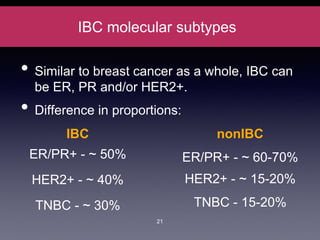
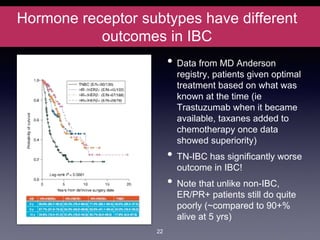
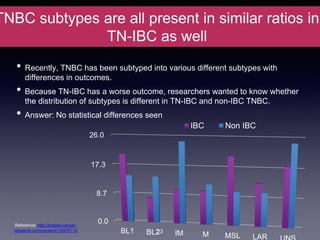
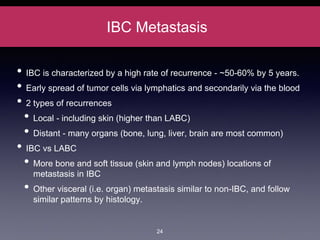
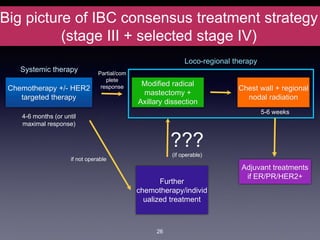
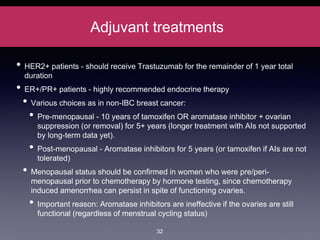
Inflammatory breast cancer (IBC) is an aggressive subtype accounting for 2-5% of breast cancer cases with distinctive clinical symptoms including rapid breast swelling and skin changes, often diagnosed without palpable lumps. It requires specialized imaging and has unique treatment protocols focused on chemotherapy and surgical interventions, with emphasis on multidisciplinary care. IBC is associated with a higher risk of recurrence and affects younger women disproportionately, necessitating awareness and accurate diagnosis to avoid misdiagnosis.