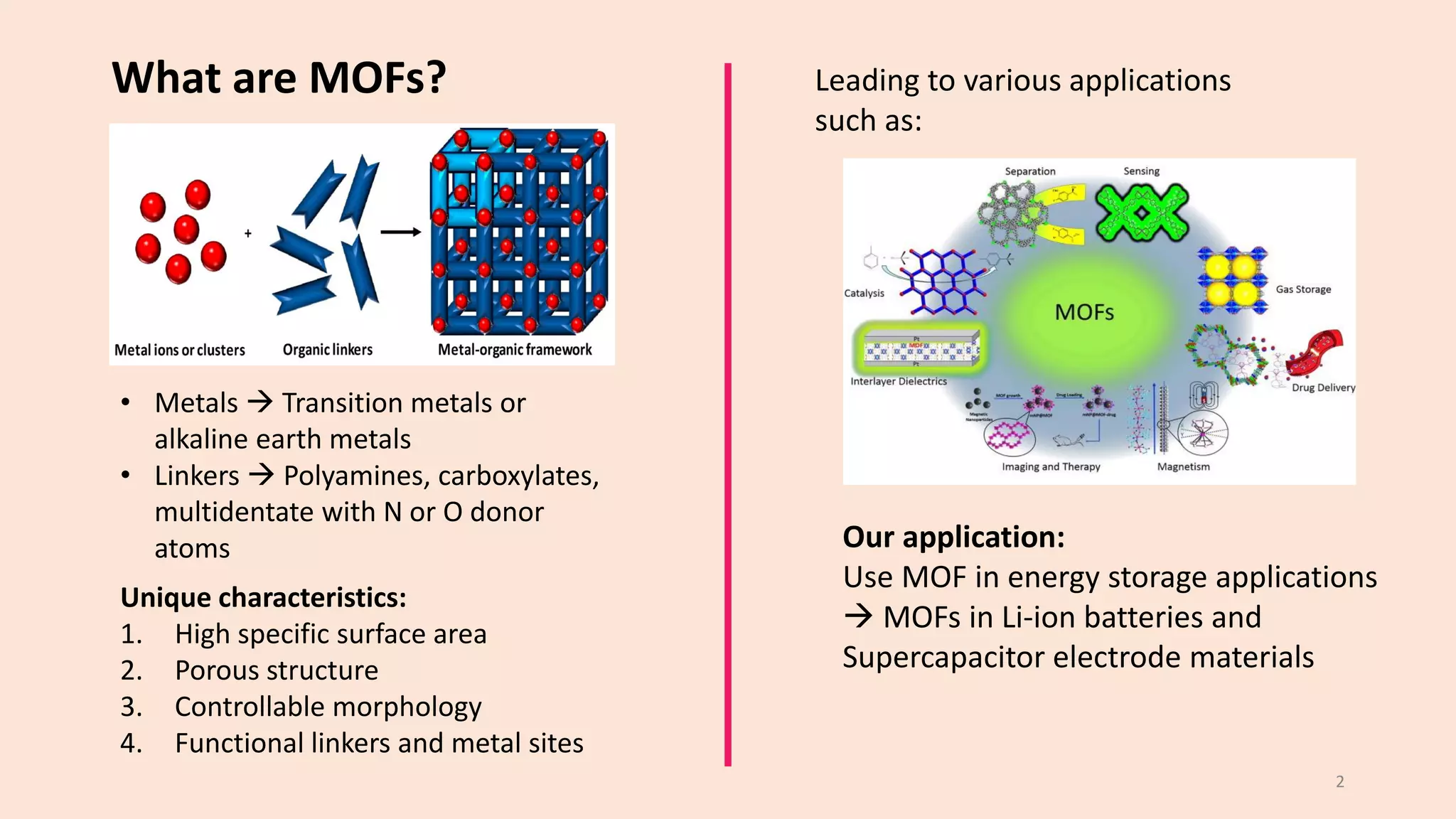
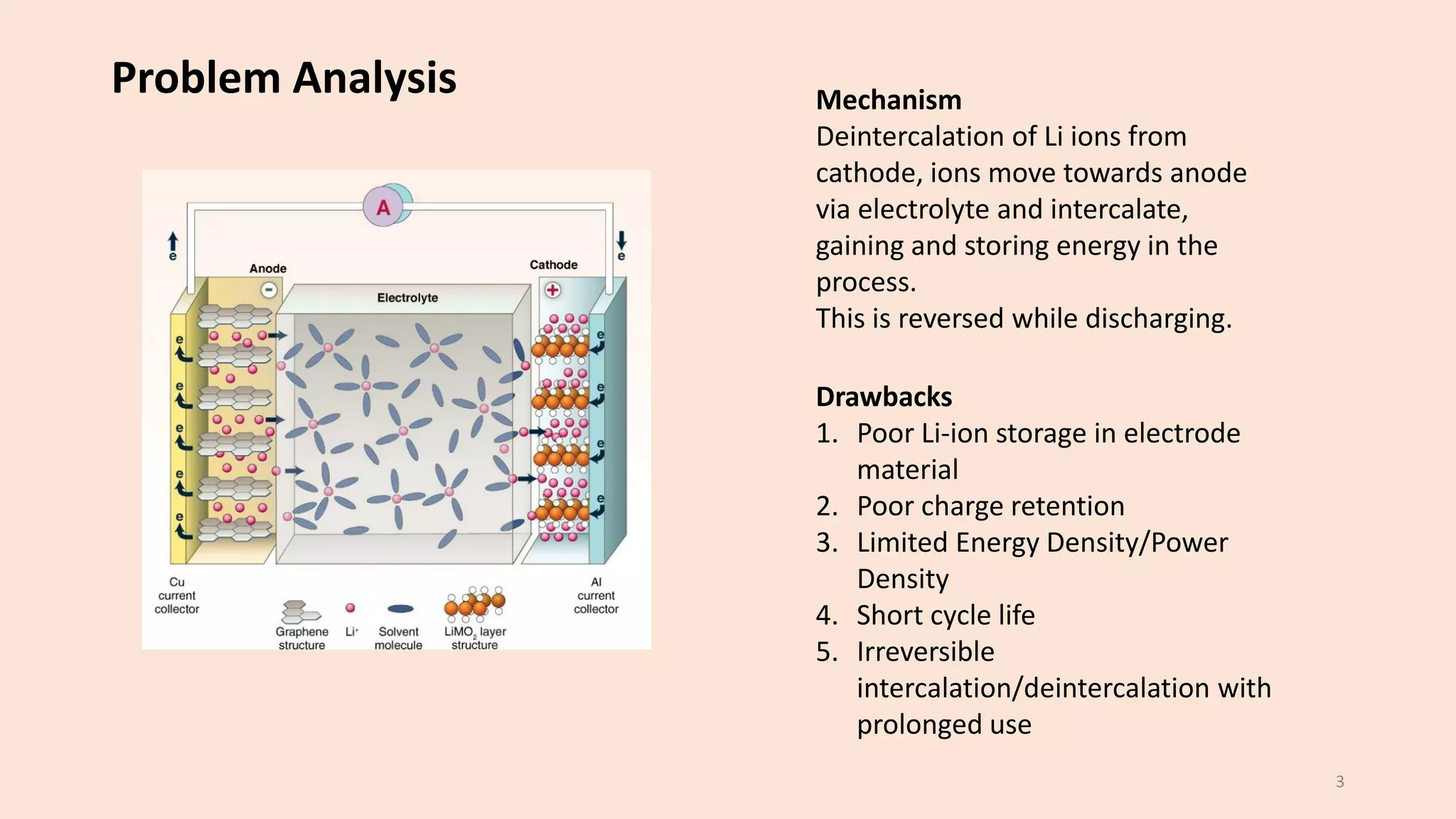
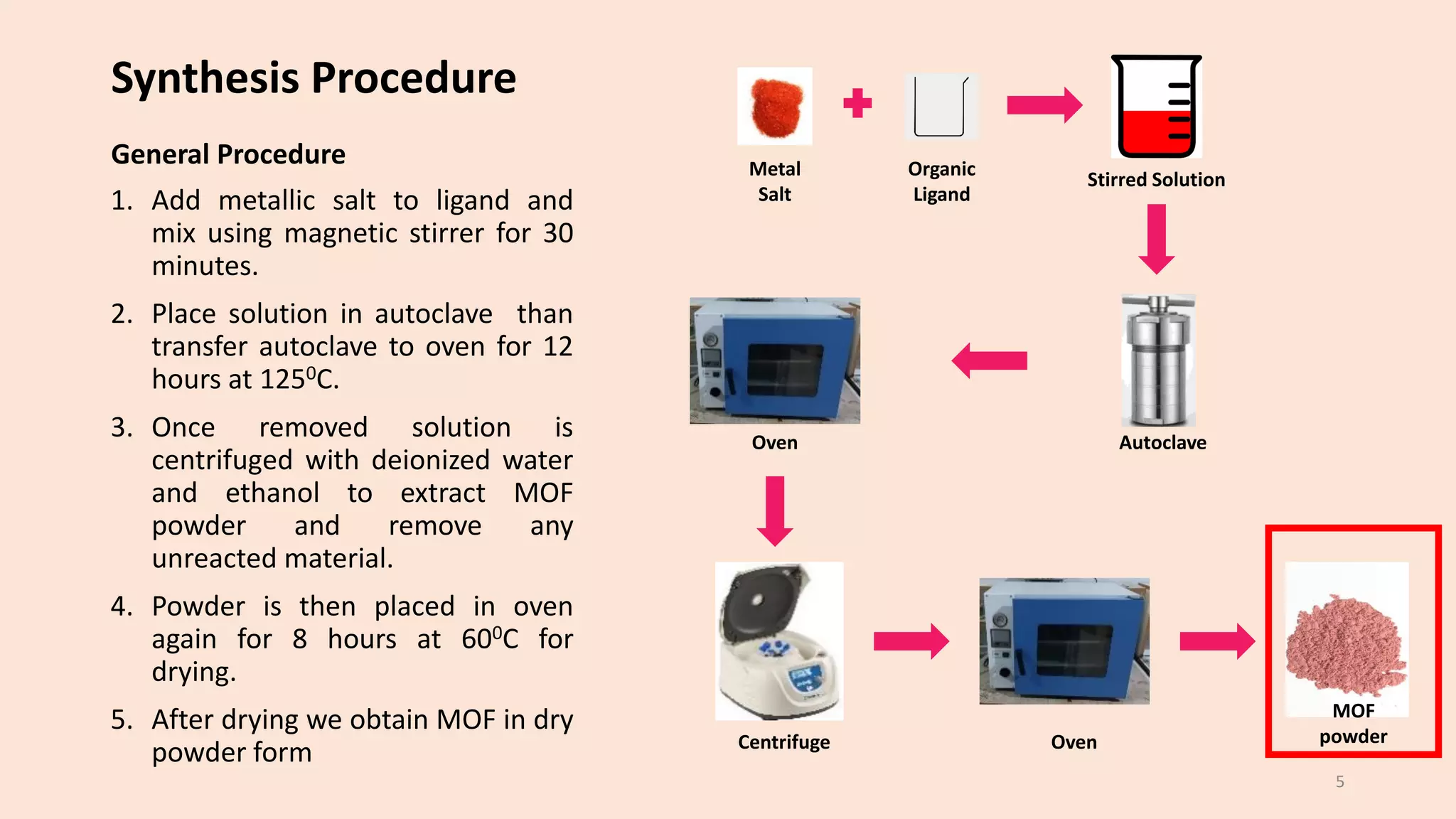
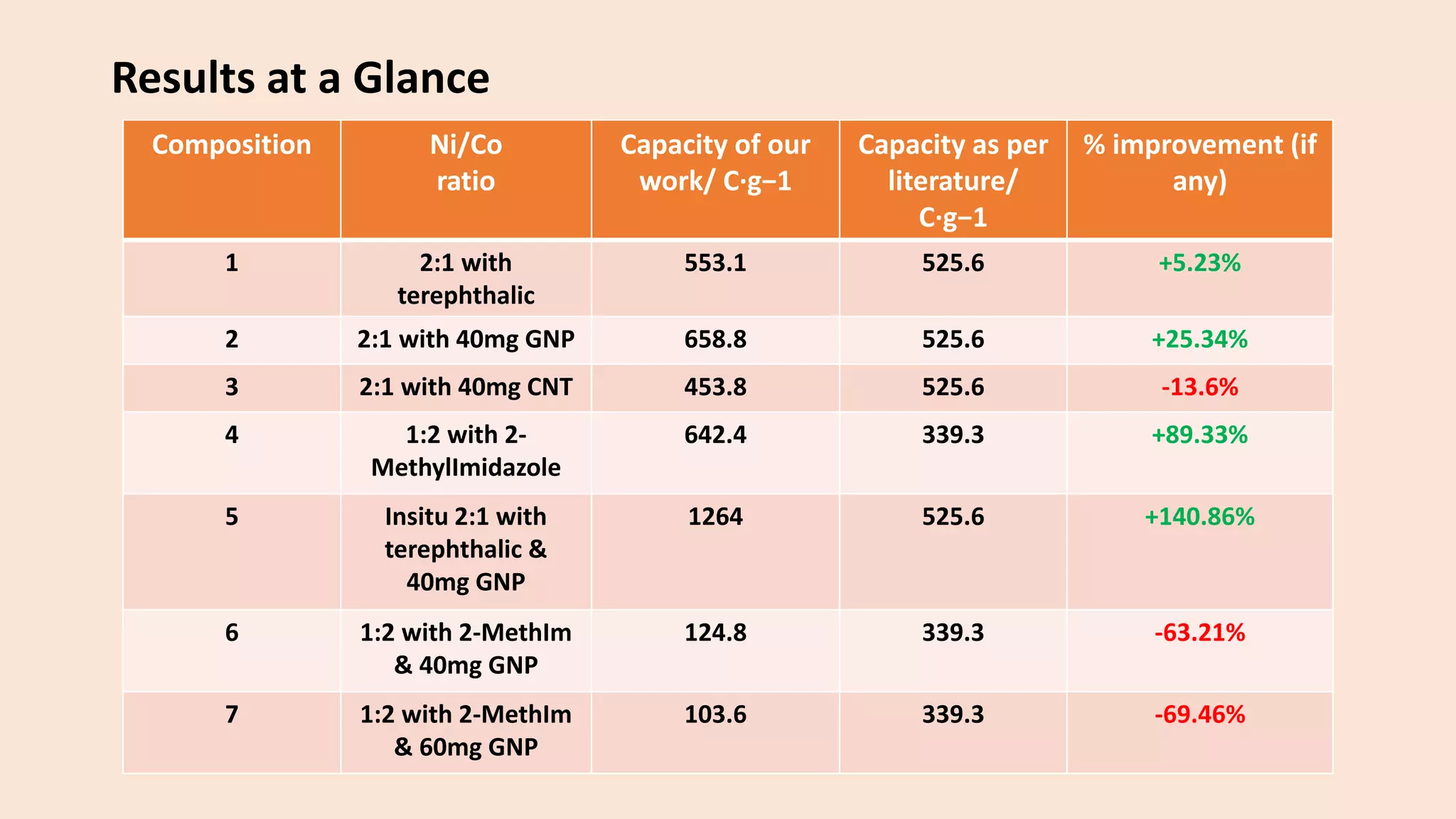
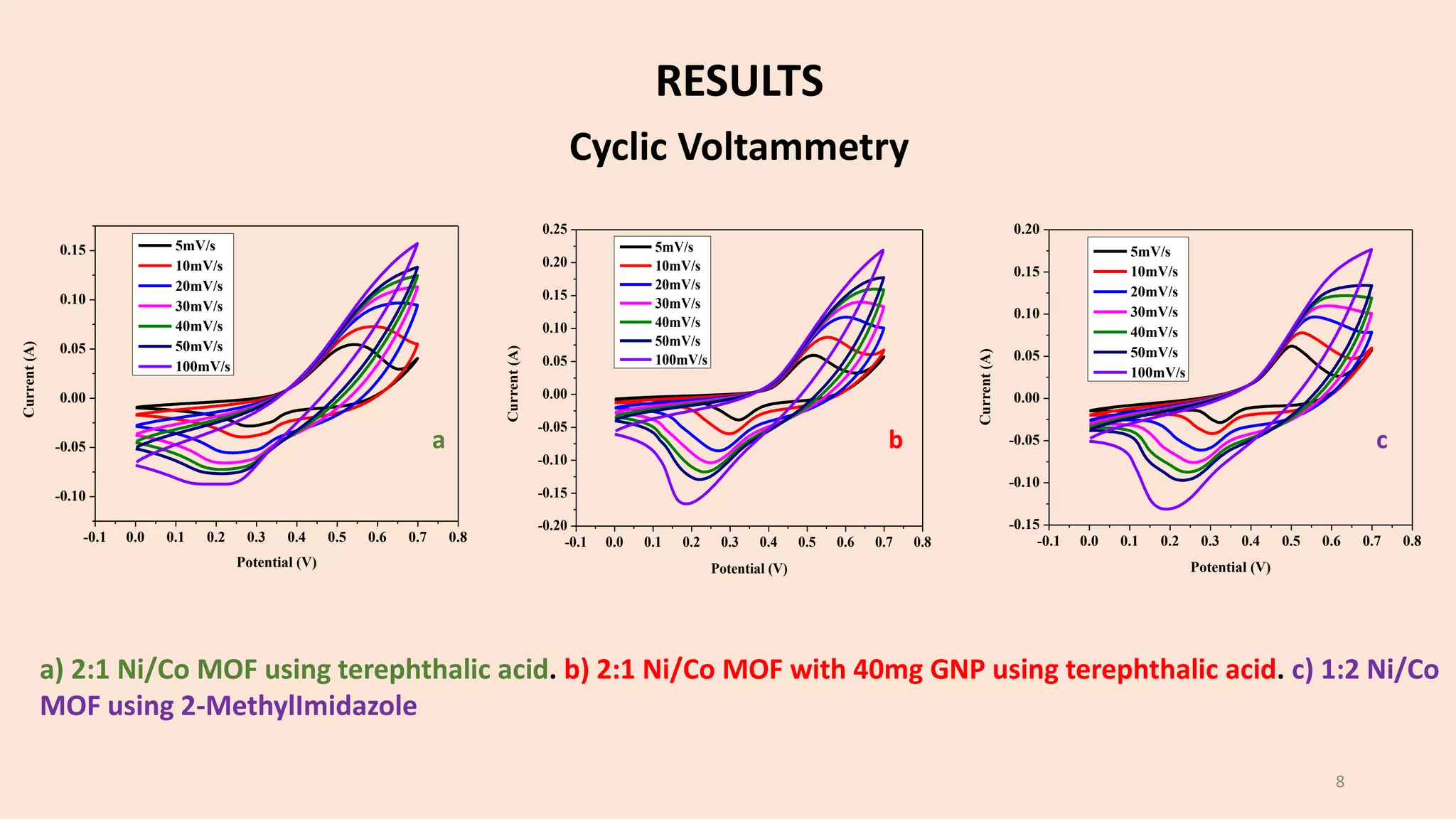
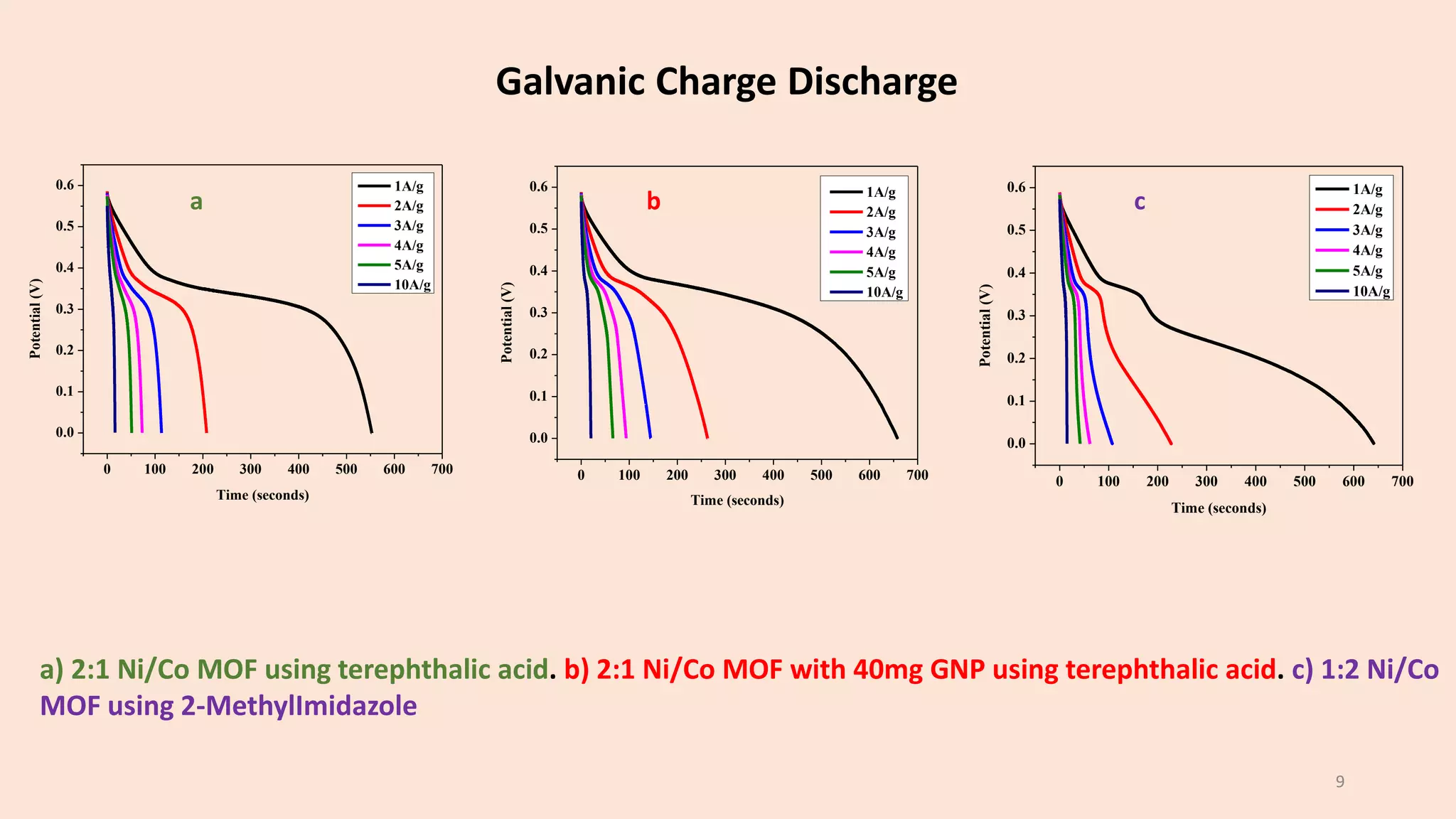
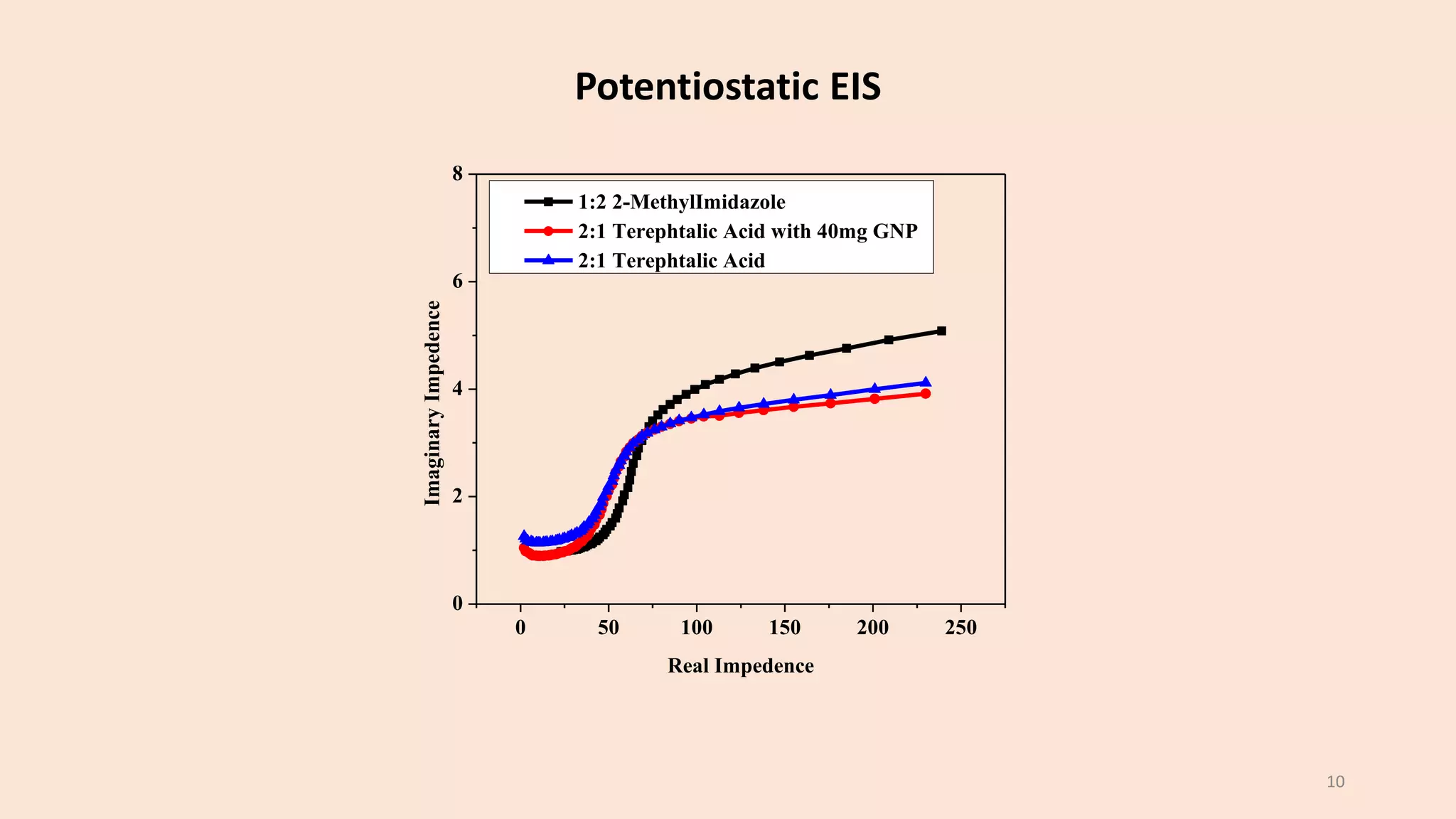
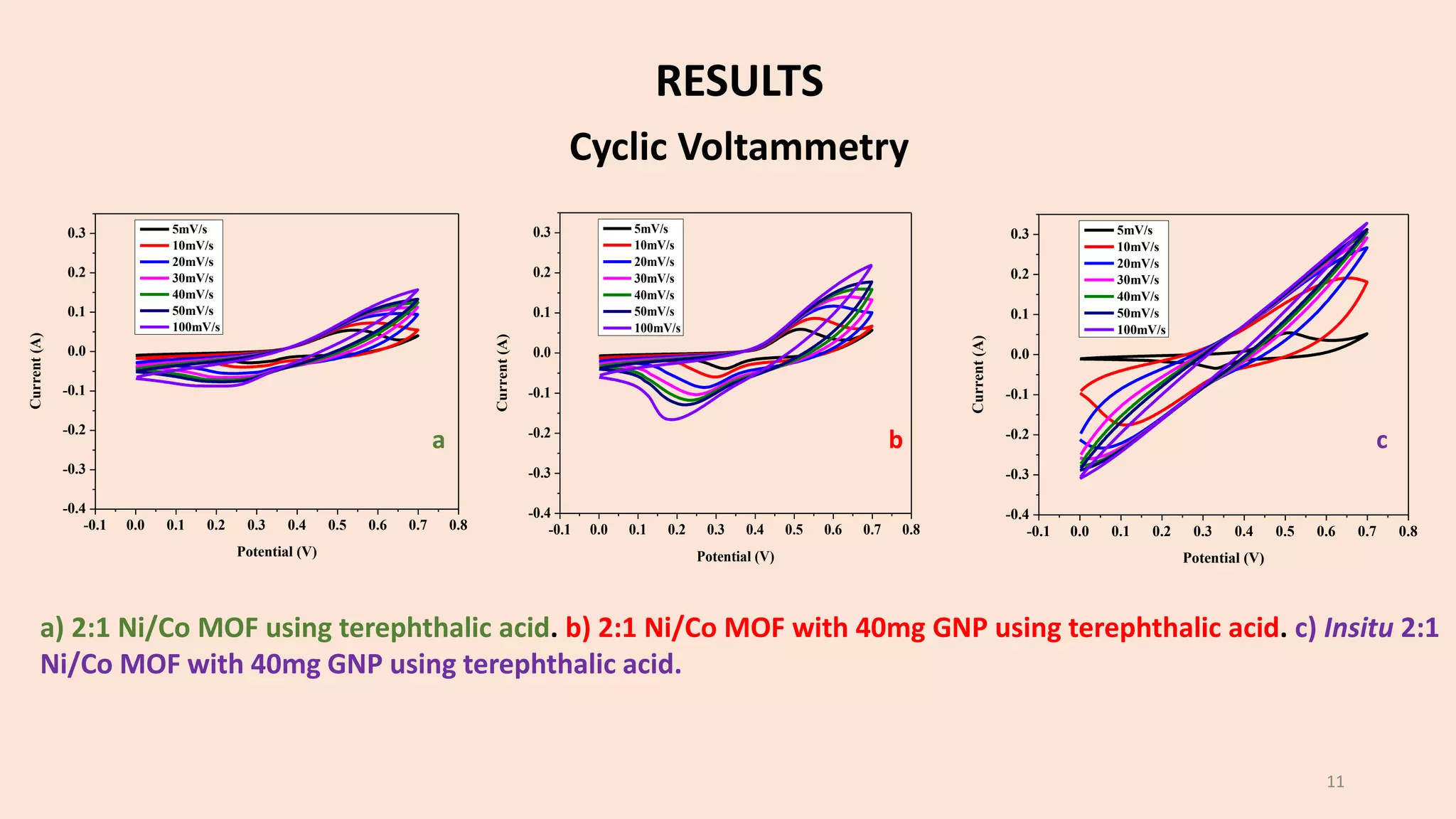
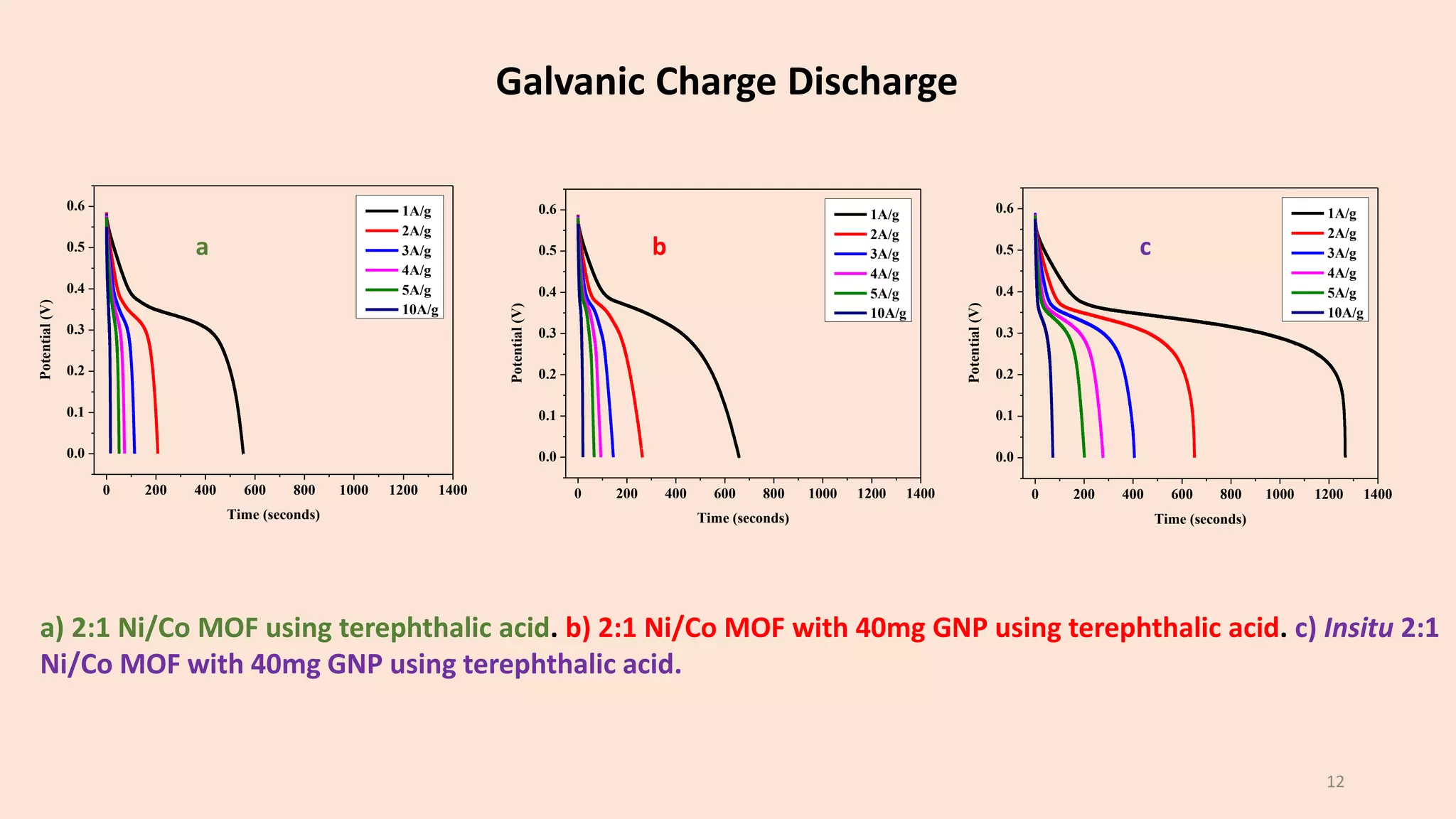
The document presents a study on the synthesis and characterization of metal-organic frameworks (MOFs) for energy storage applications, specifically in lithium-ion batteries and supercapacitors. It outlines the synthesis process, various compositions tested, and the performance results, highlighting significant improvements in capacity with certain compositions. The study aims to overcome existing limitations in electrode materials by using MOFs to enhance energy density, power density, and overall performance.