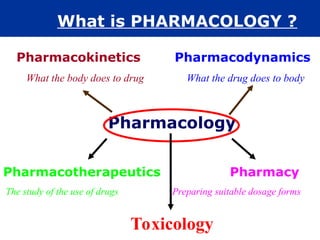
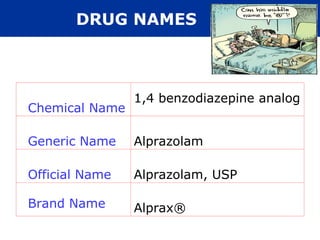
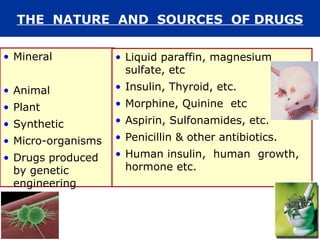
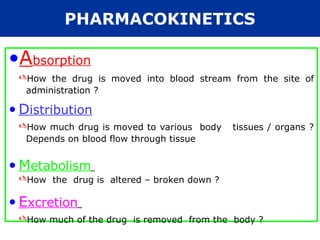
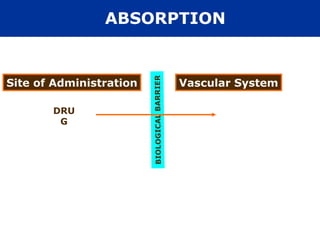
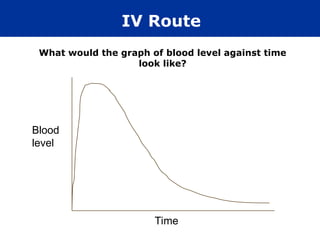
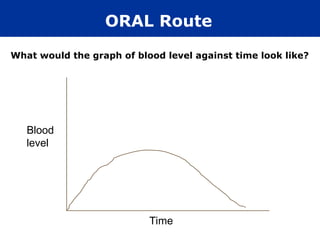
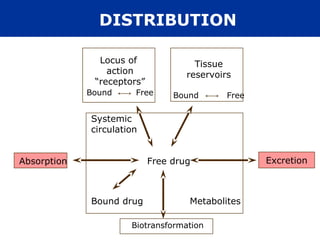
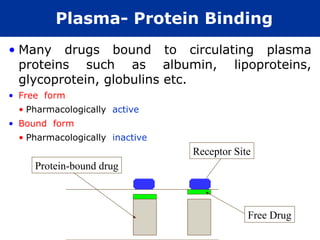
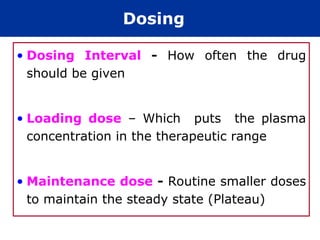
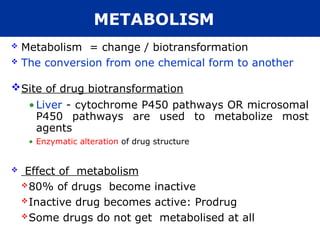
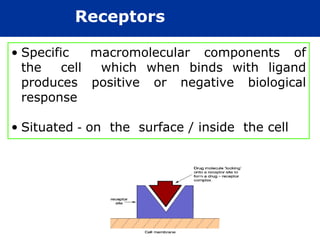
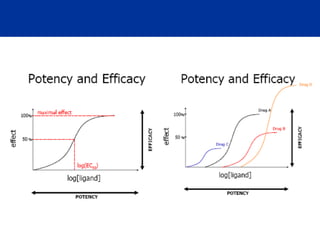
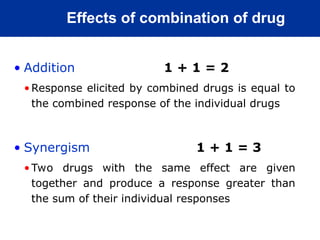
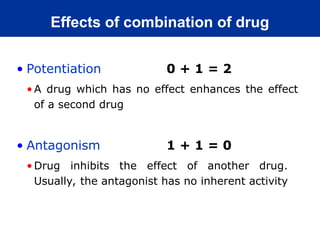
The document provides an extensive overview of pharmacology, including concepts such as pharmacokinetics and pharmacodynamics, drug dosage forms, routes of administration, and metabolism. It details the mechanisms of how drugs affect the body, their classification, and the importance of understanding drug interactions, half-life, and therapeutic effects. Additionally, it discusses considerations for drug use in different populations, including pregnant and geriatric patients.































![• The rational pharmacological treatment of
any patient requires adequate knowledge
about :
The disease process,
Pharmacodynamic properties of the
drug(s) selected, and
The individual’s handling of the drug(s)
[pharmacokinetics].
FUNDAMENTALS OF PHARMACOLOGY](https://image.slidesharecdn.com/pharmacology-120806022529-phpapp01-250120212800-be4efb62/85/pharmacology-12080602266529-phpapp01-ppt-74-320.jpg)