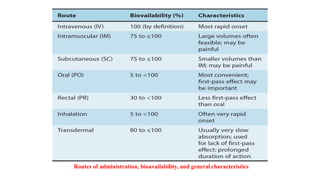
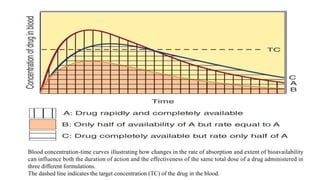
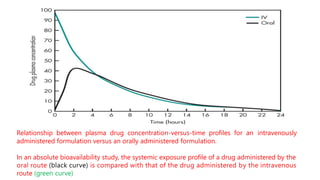
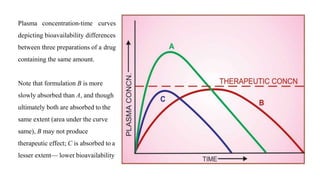
The lecture by Dr. Awais Irshad covers key pharmacology concepts such as bioavailability, bioequivalence, half-life, and drug dosing strategies. It details the factors affecting drug absorption and metabolism, including routes of administration, first-pass elimination, and physicochemical properties. Additionally, it discusses the significance of loading and maintenance doses in achieving effective drug concentrations in the plasma.