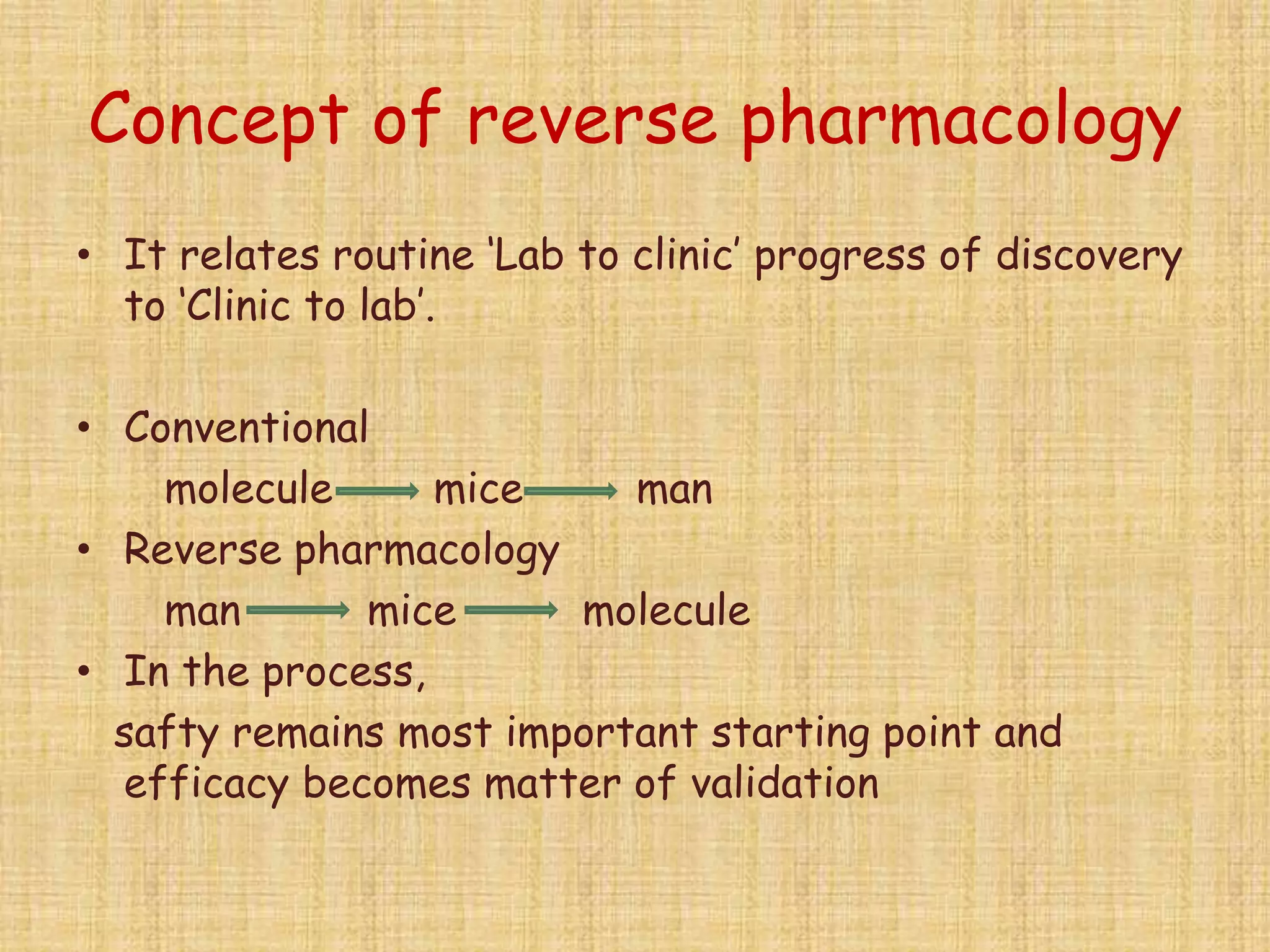
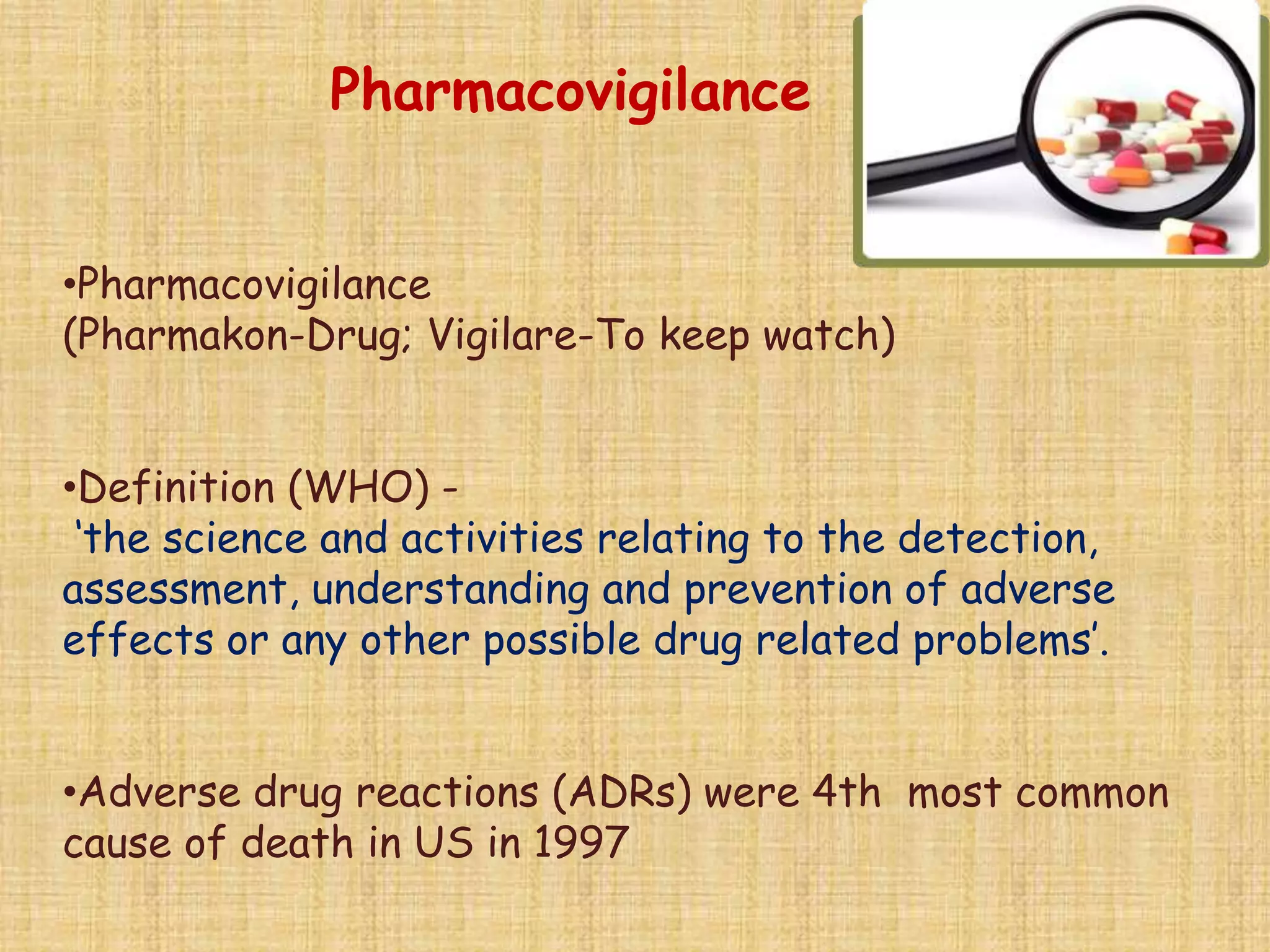
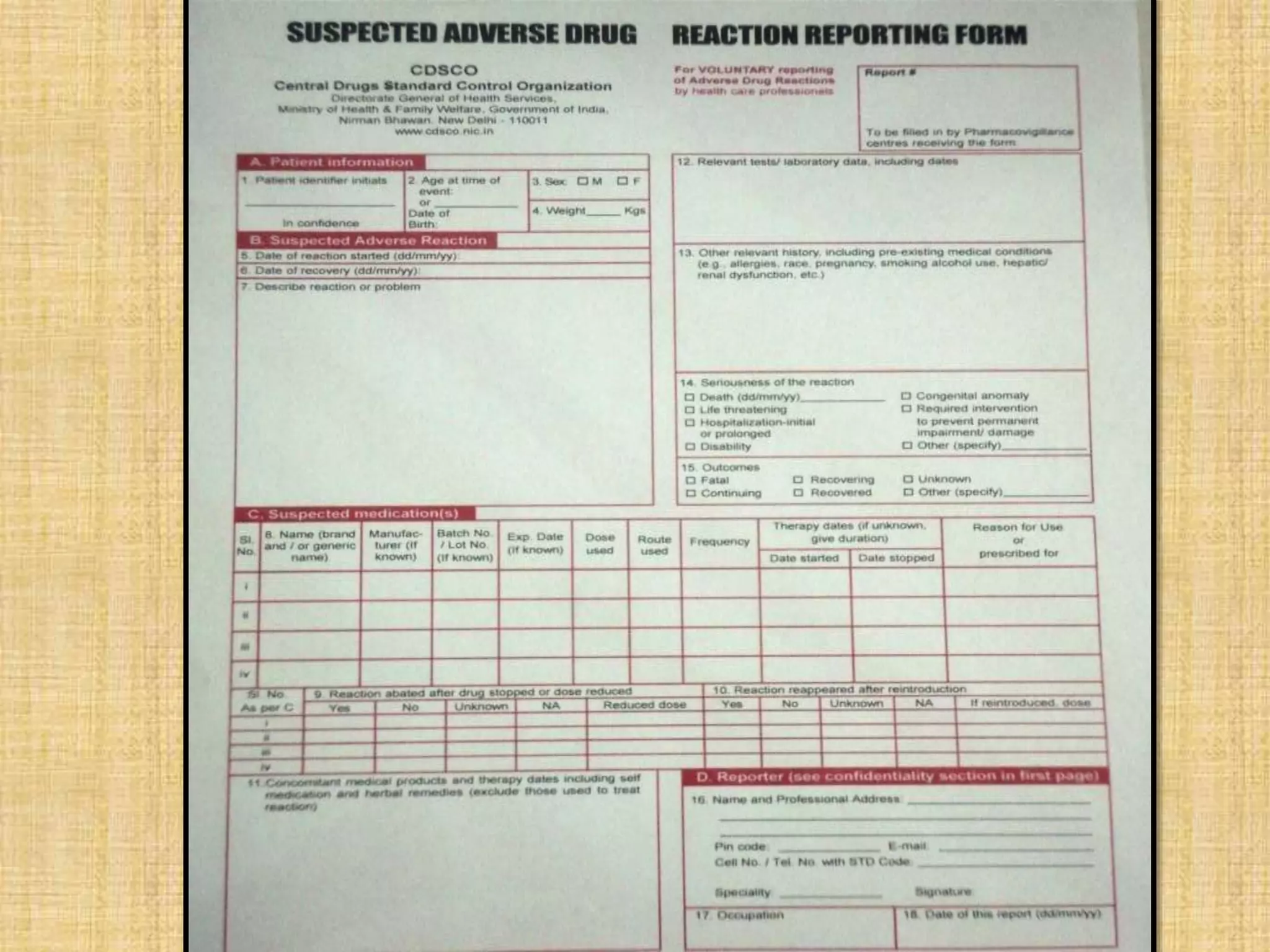
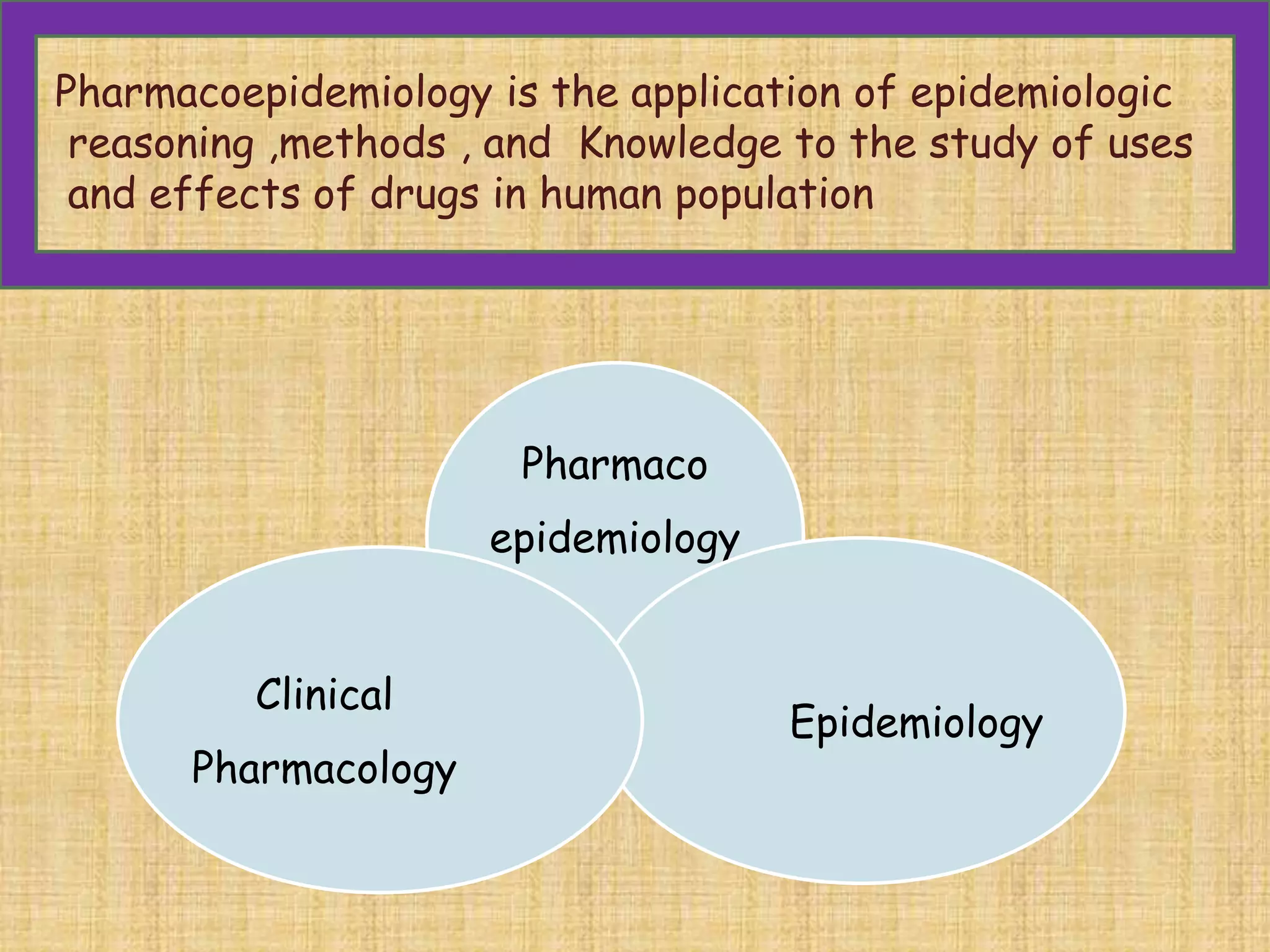
This document provides an overview of the scope of pharmacology. It discusses the history and evolution of pharmacology from materia medica and early pharmacy to its modern academic, industrial and research applications. Key areas of pharmacology discussed include drug development process, clinical pharmacology, special domains like pharmacovigilance, pharmacoeconomics and emerging areas like pharmacogenomics. The document outlines the past, present and future scope of pharmacology and how it aims to advance human health through rational and safe use of medicines.