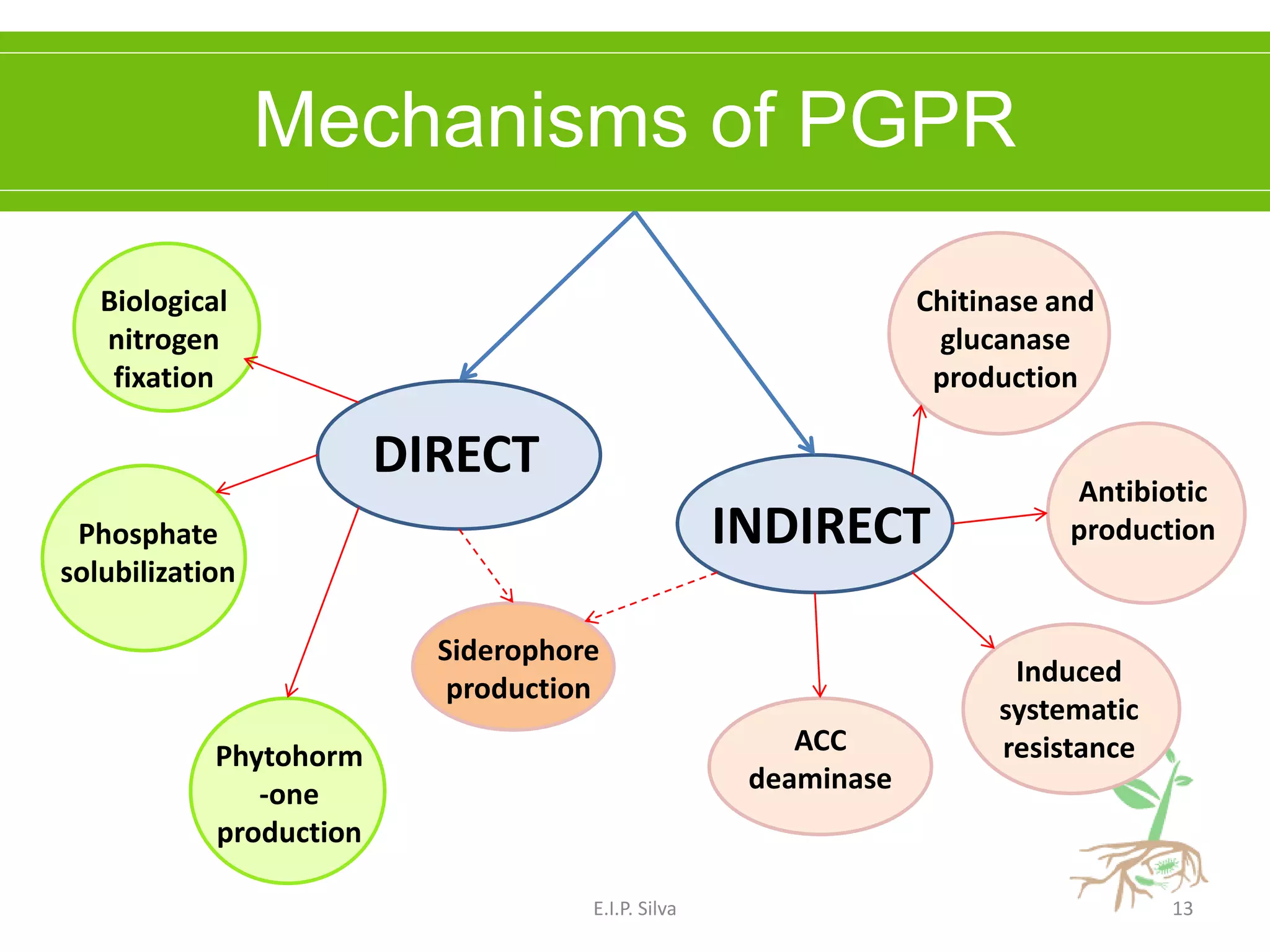
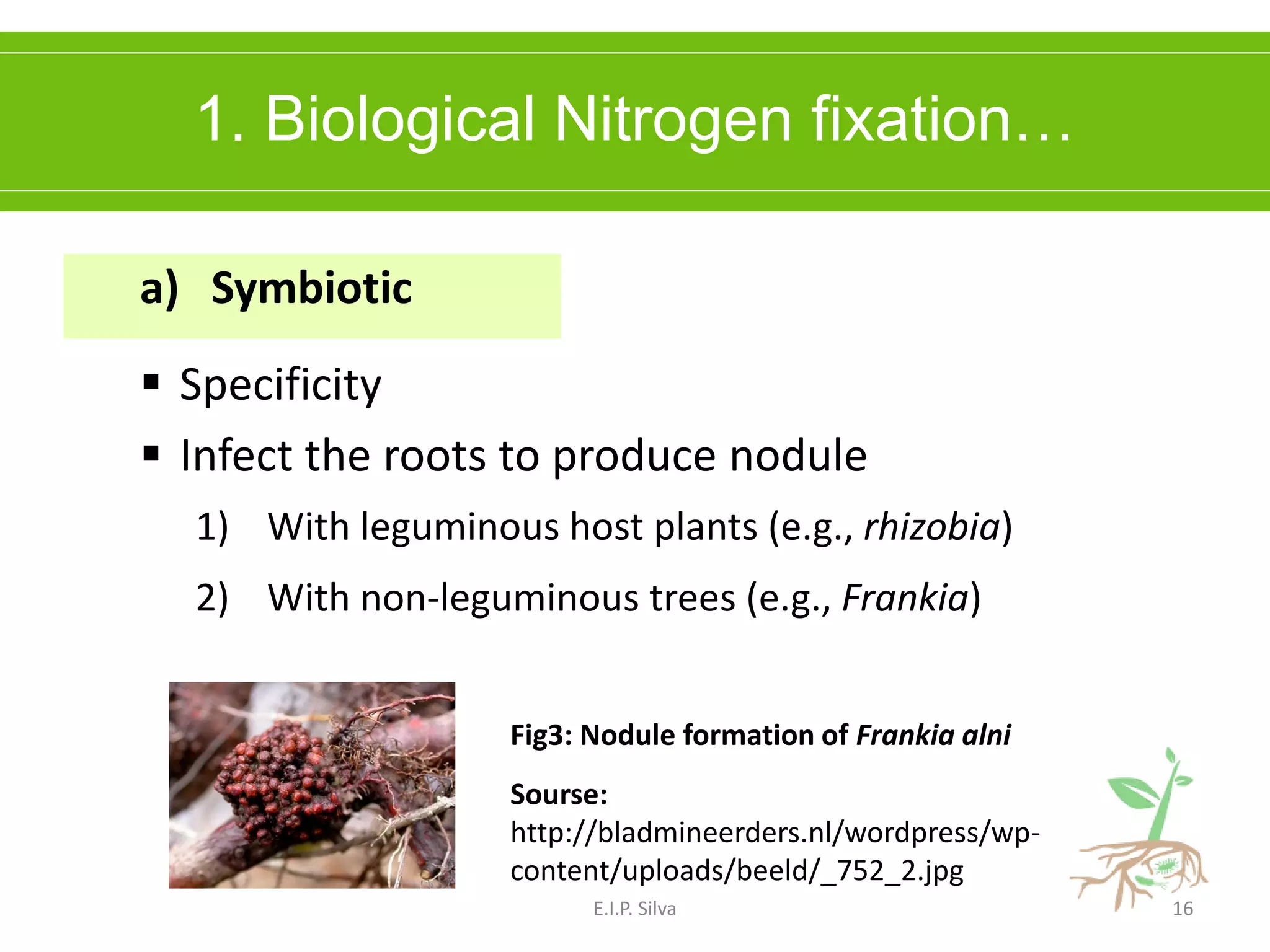
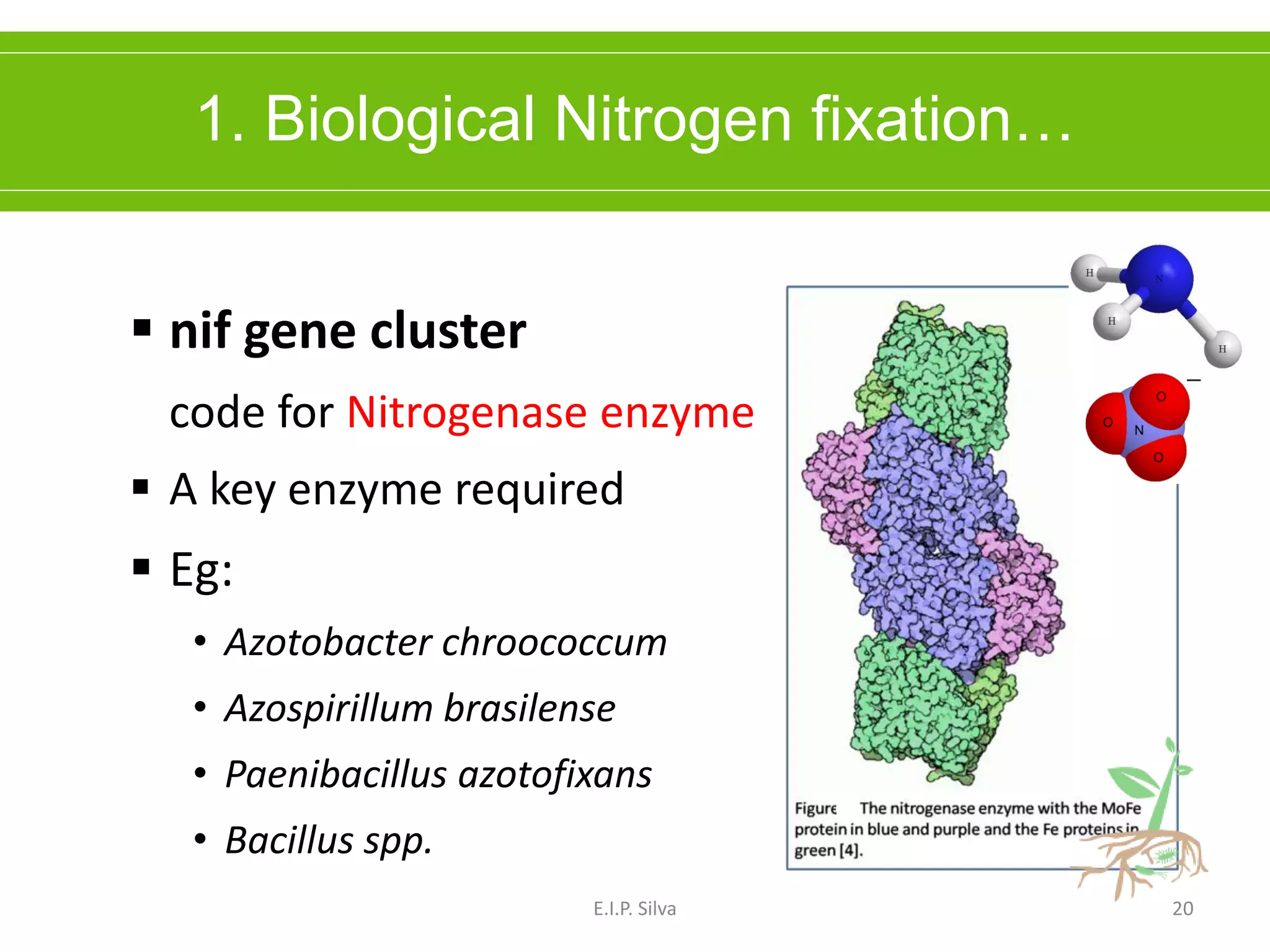
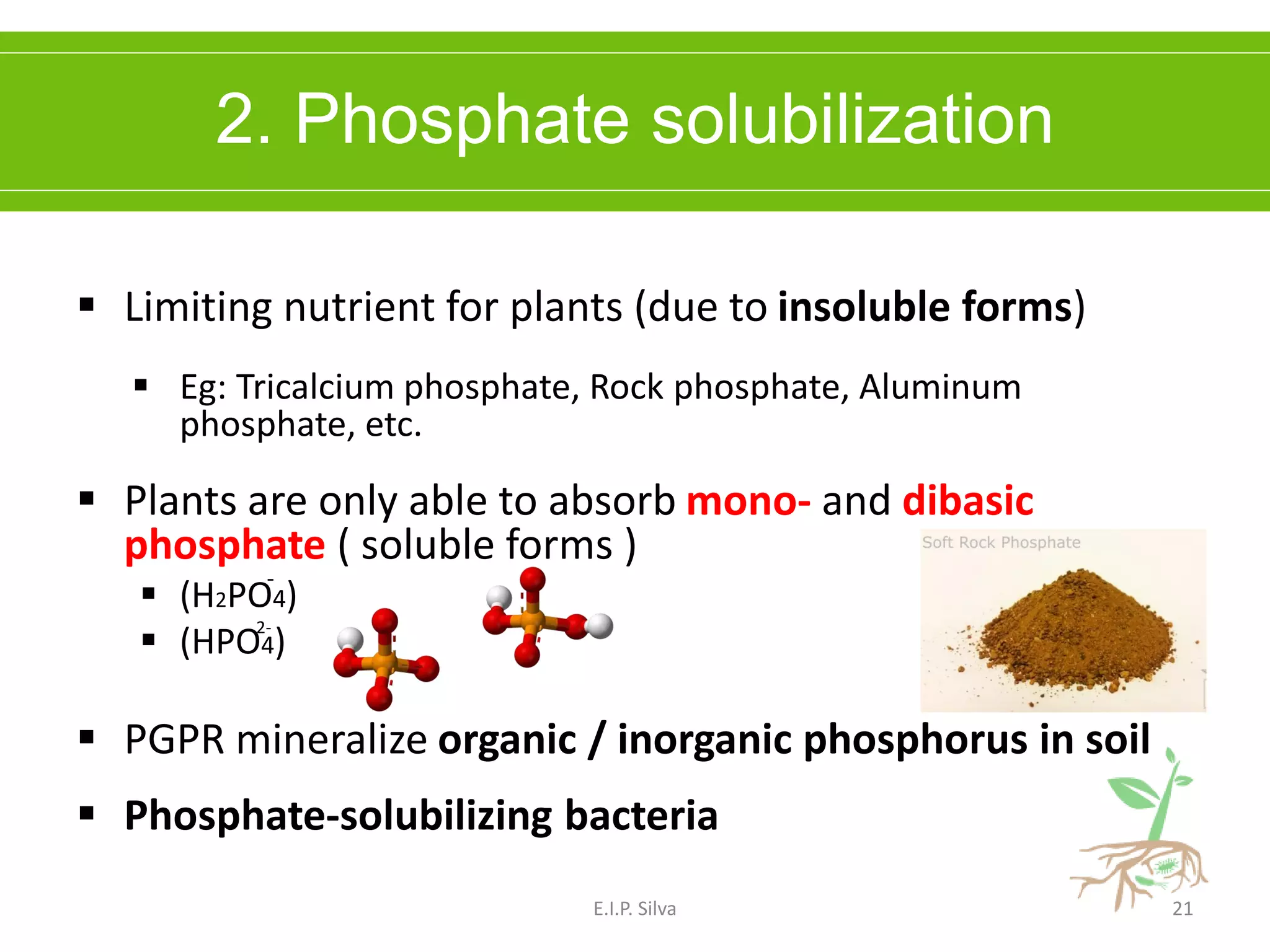
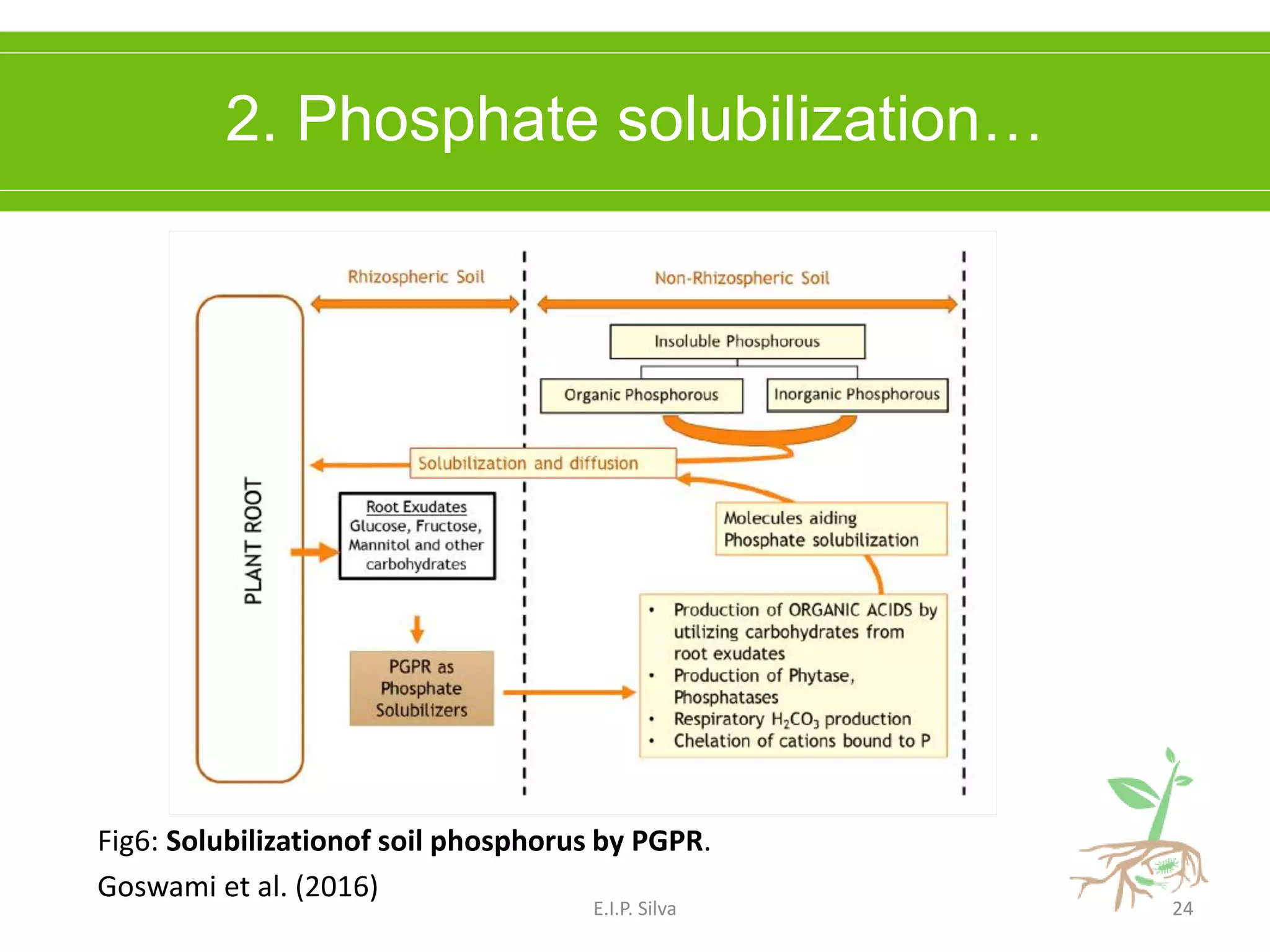
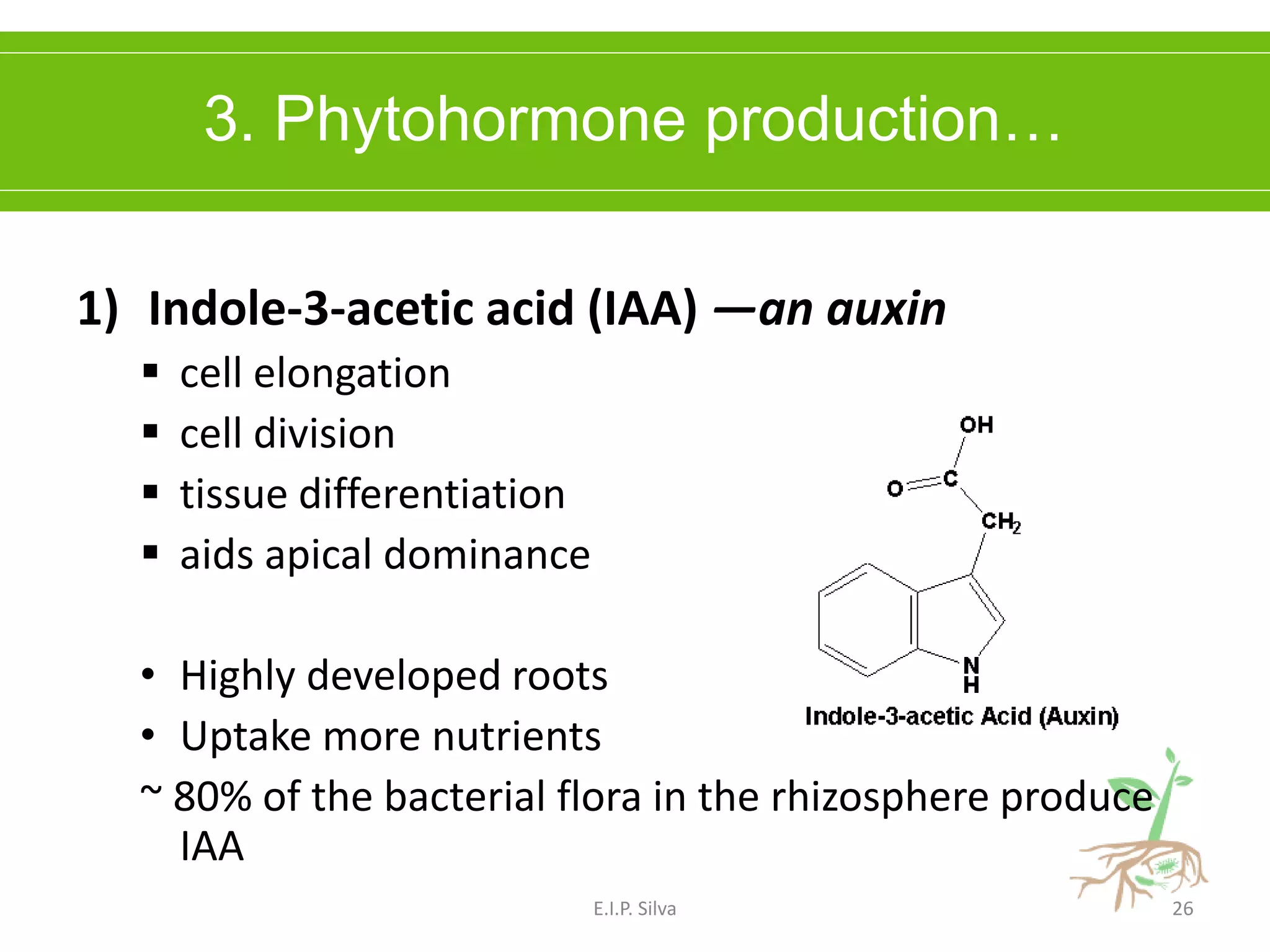
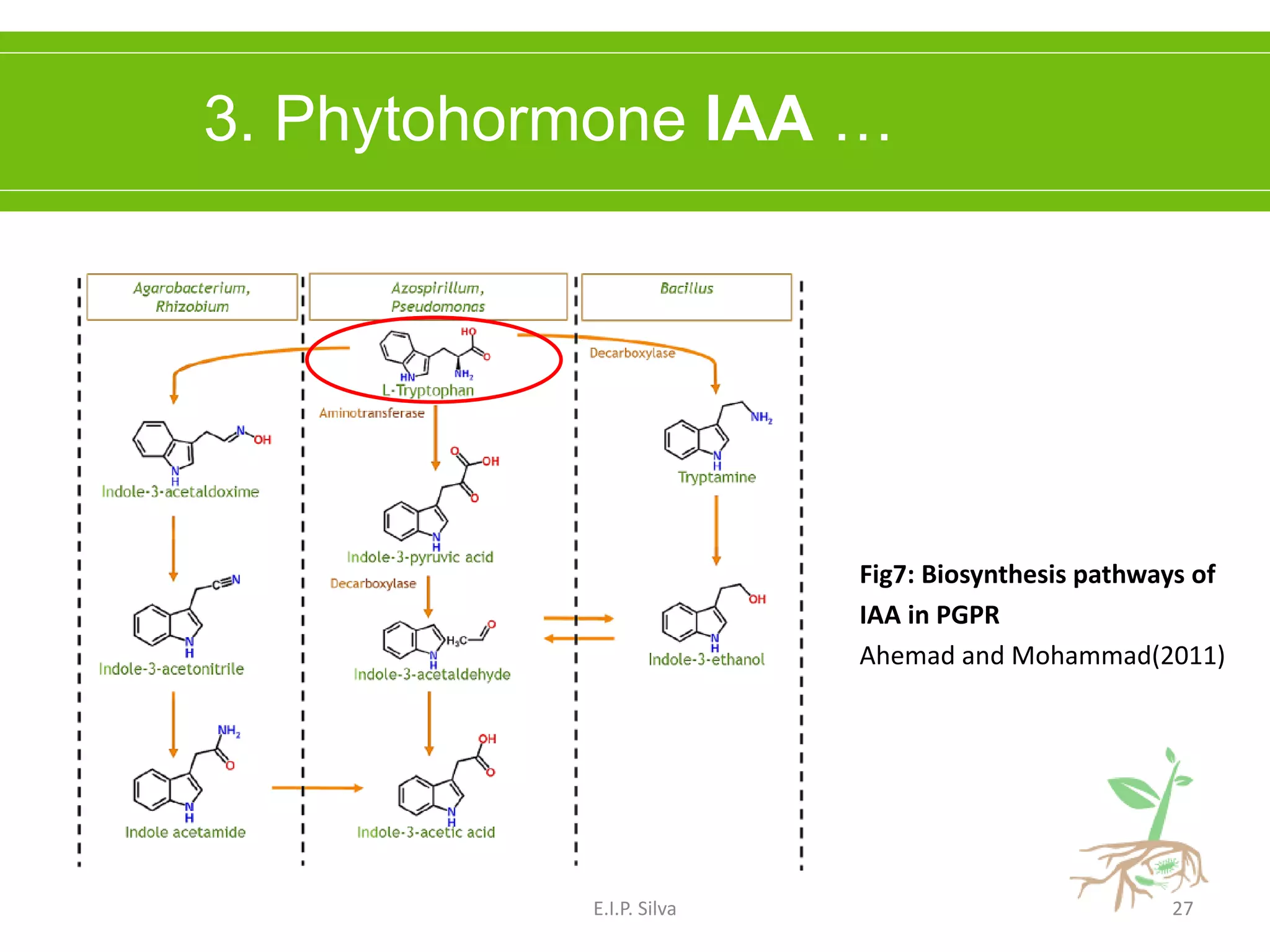
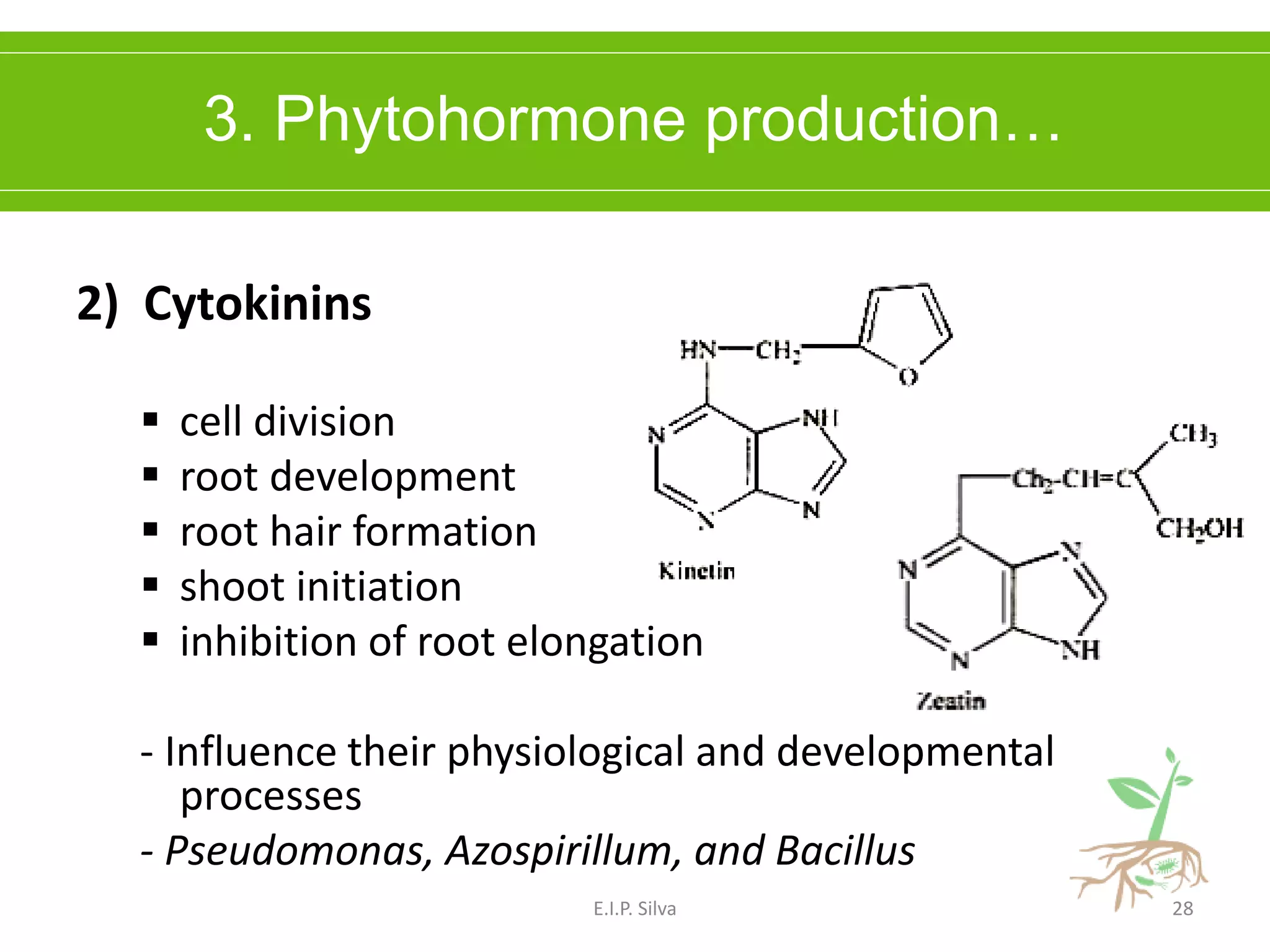
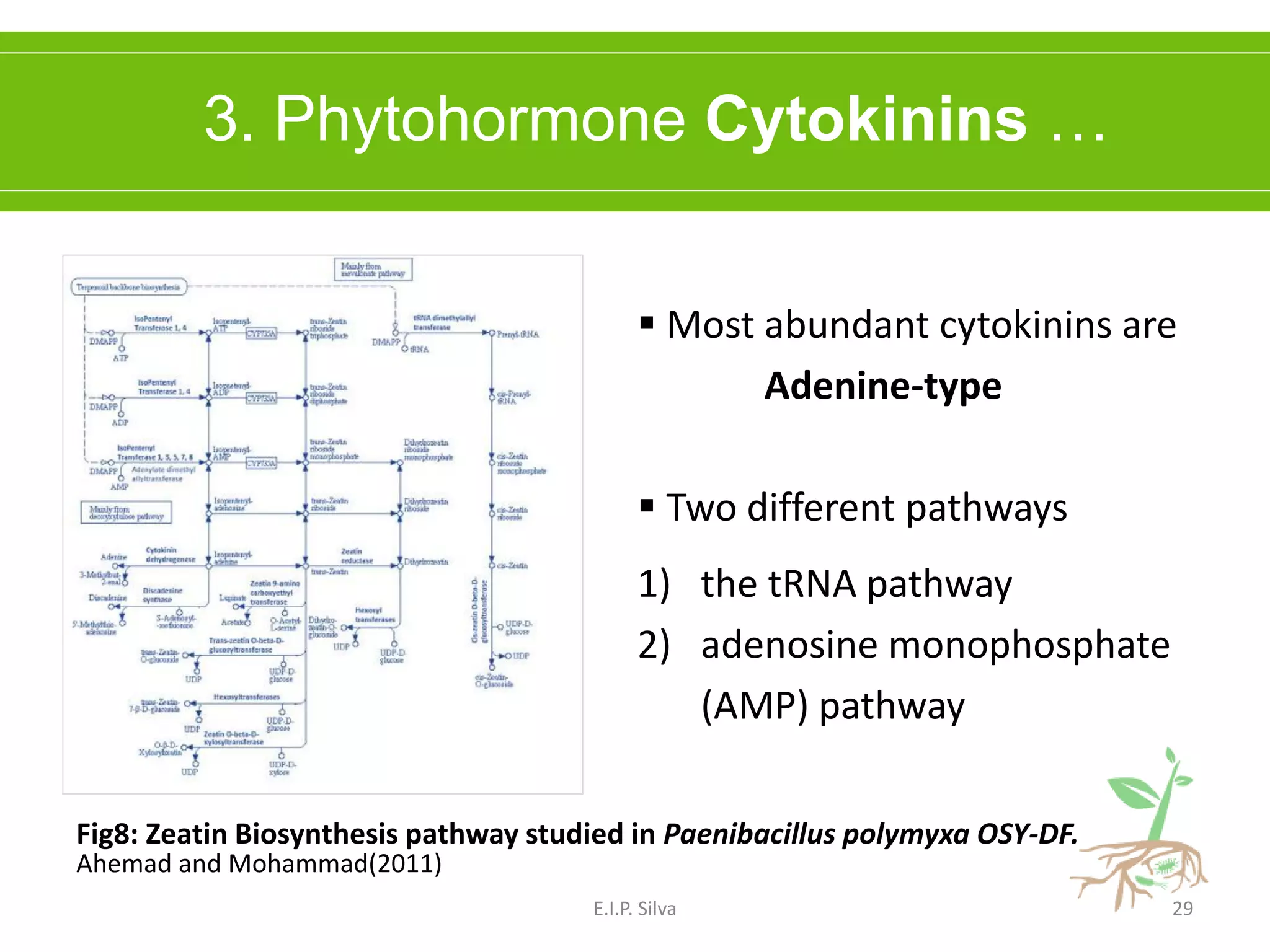
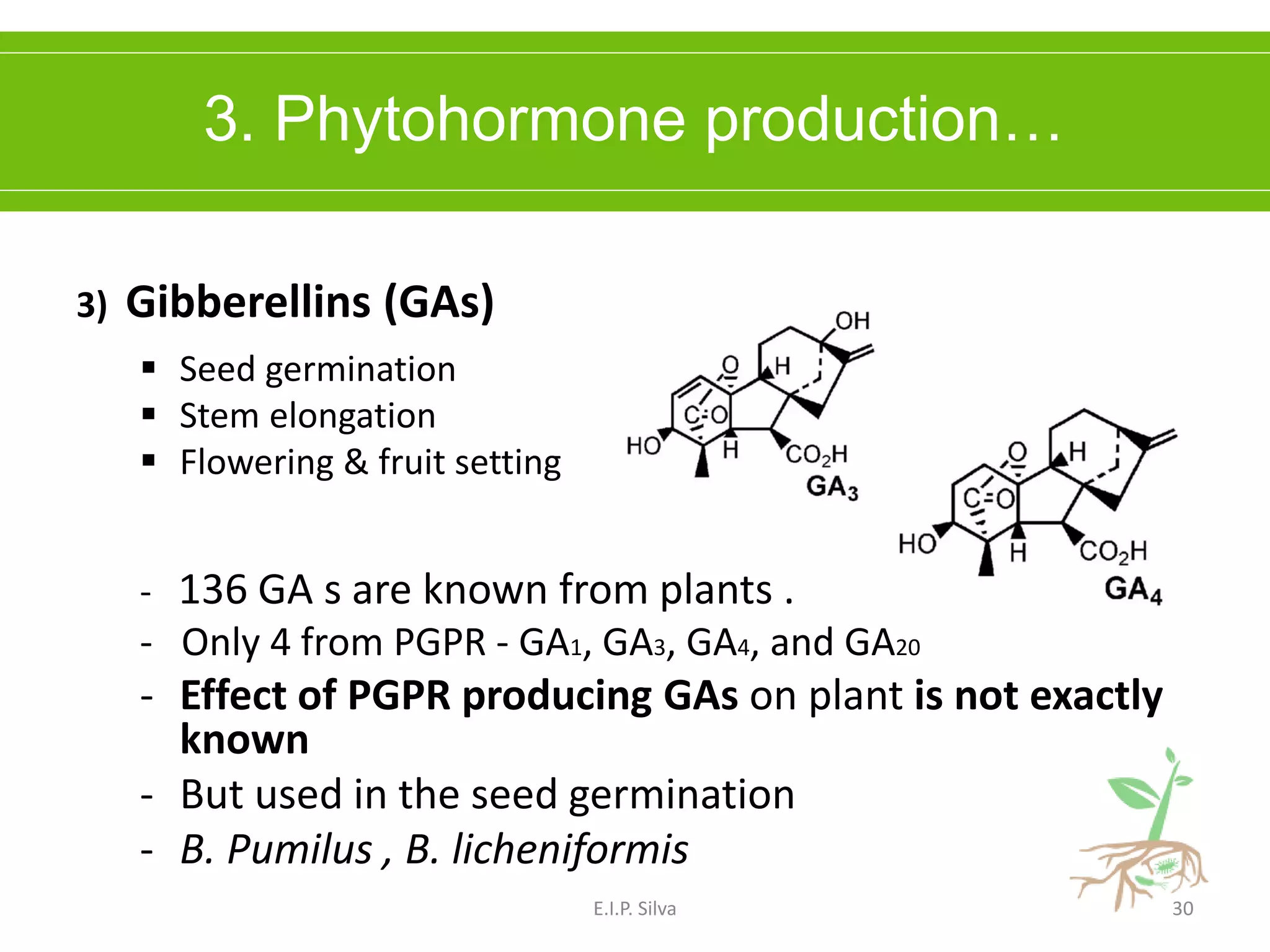
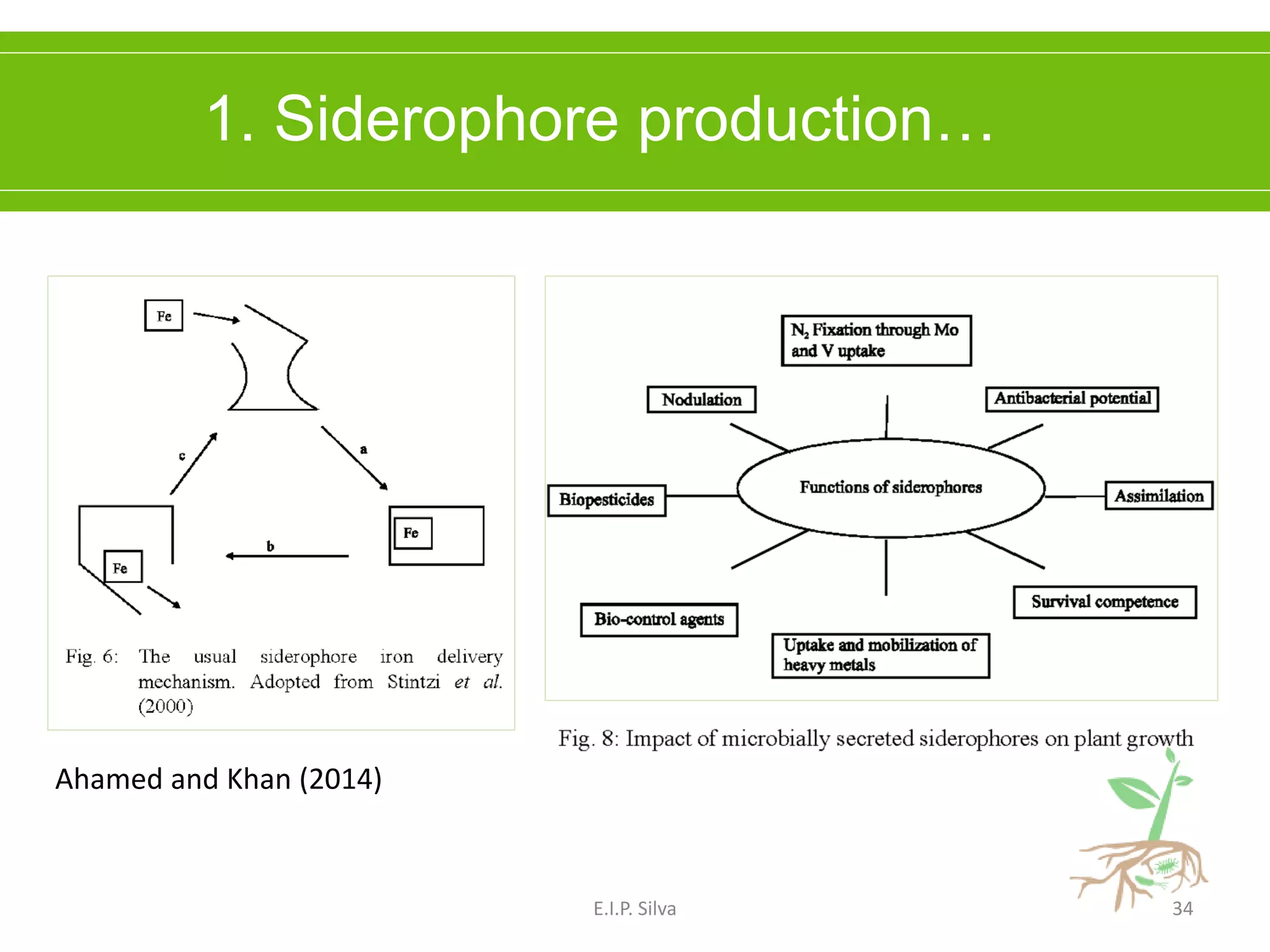
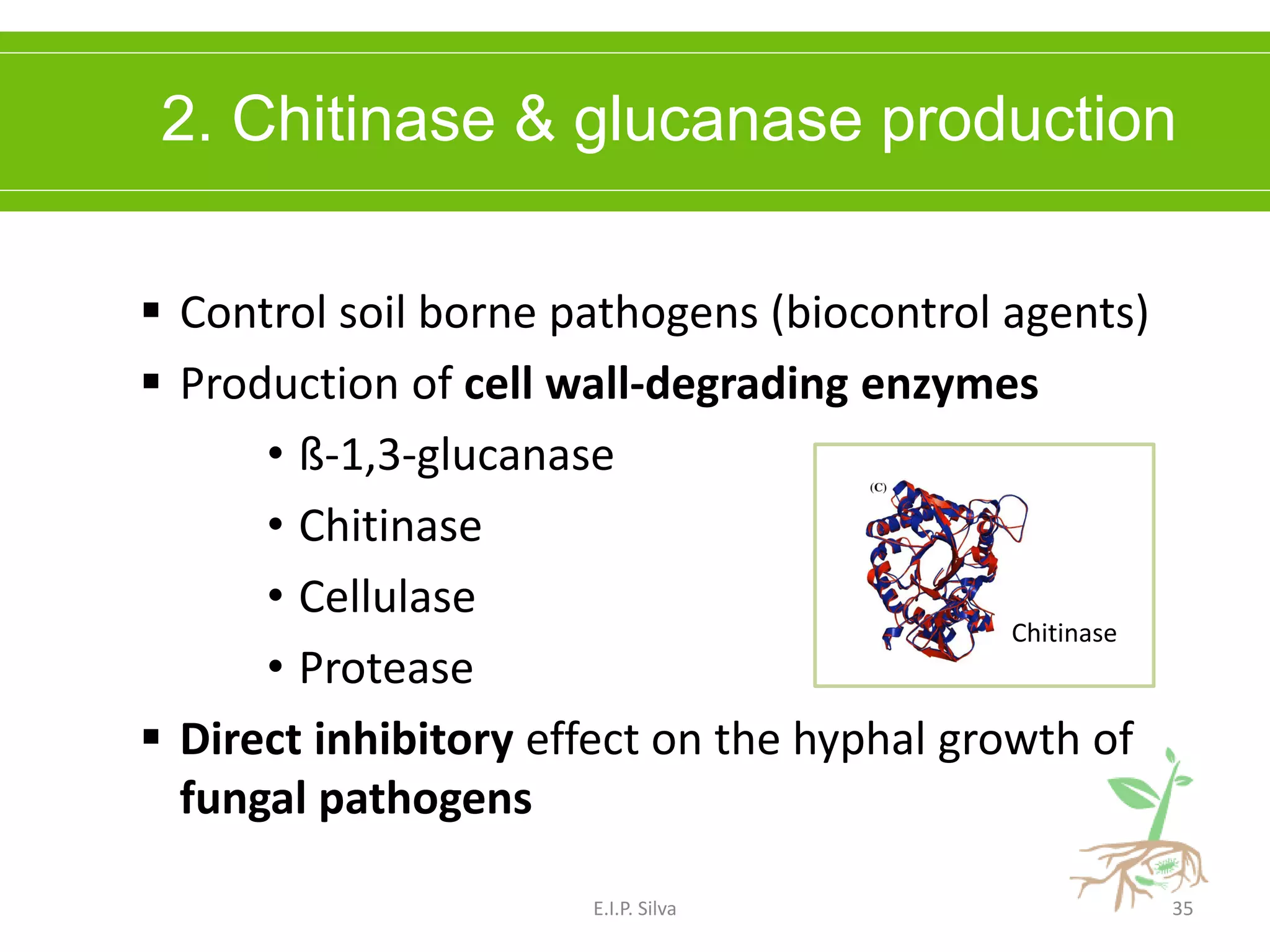
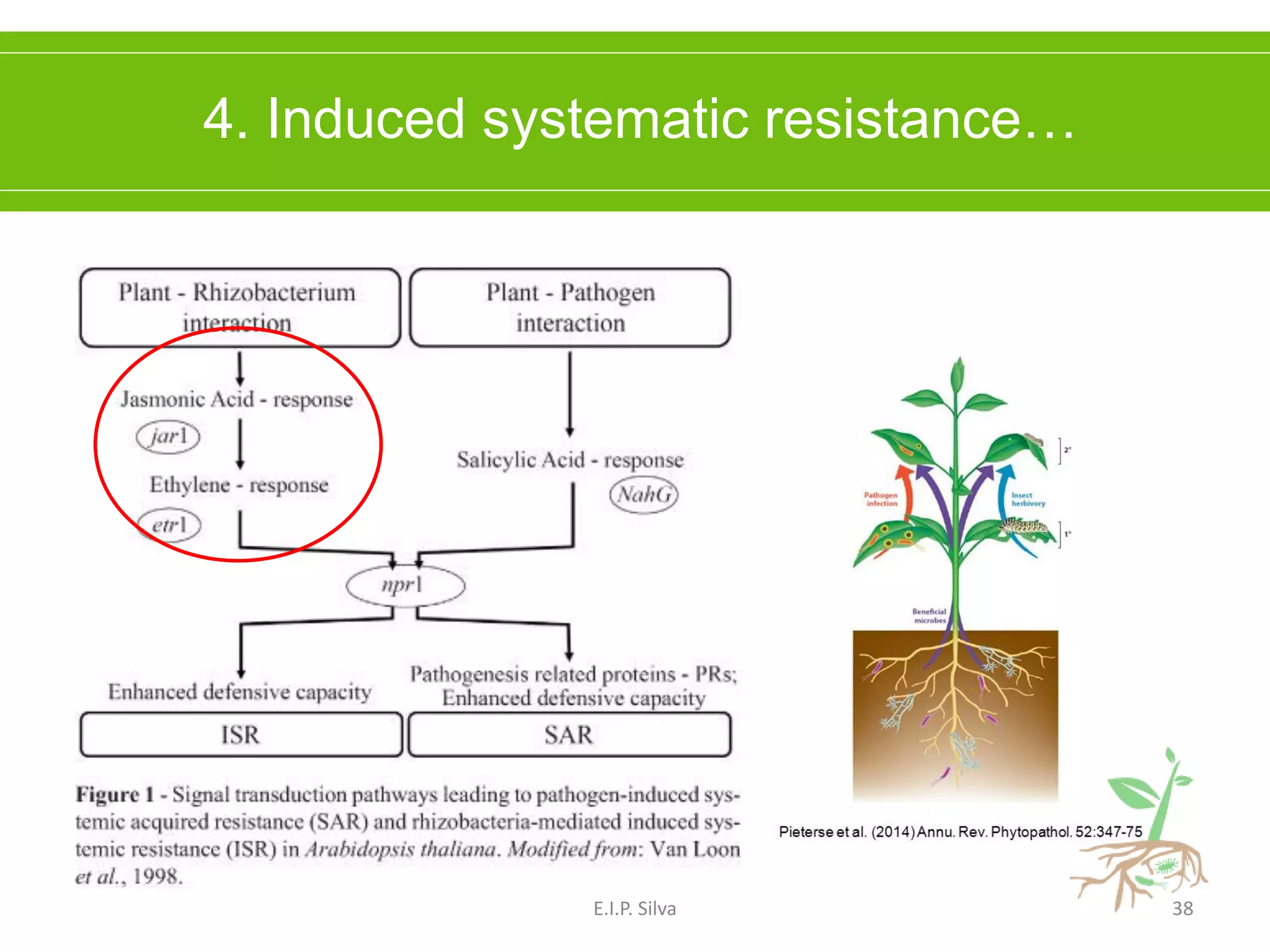
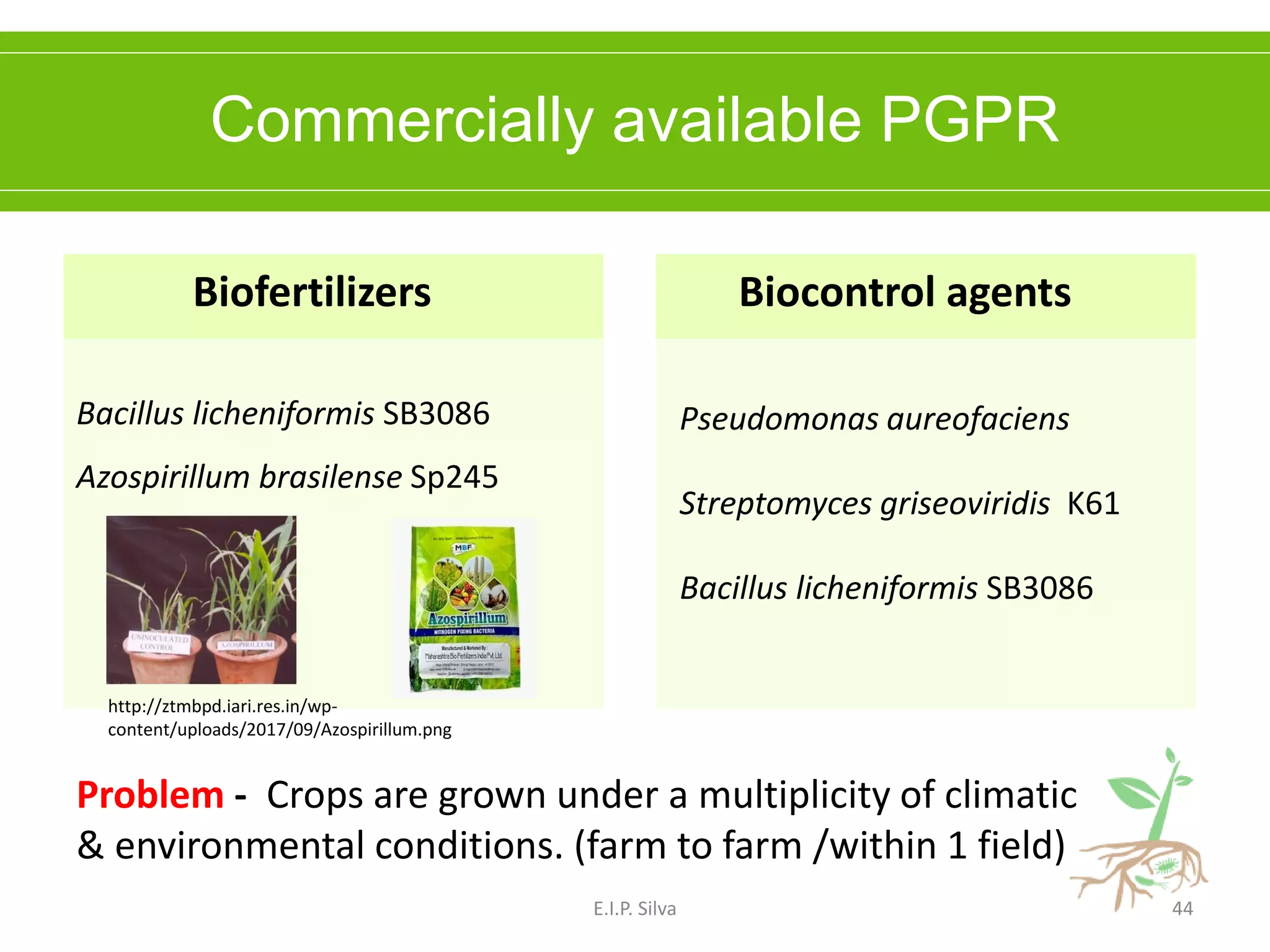
This document provides an overview of plant growth promoting rhizobacteria (PGPR). It discusses that PGPR are a group of soil bacteria that colonize plant roots and enhance plant growth directly or indirectly. Direct mechanisms include biological nitrogen fixation, phosphate solubilization, phytohormone production, siderophore production, and antibiotic production. Indirect mechanisms include inducing systemic resistance in plants, production of lytic enzymes, and stress tolerance effects. The document reviews the specific mechanisms of several important PGPR functions and commercially available PGPR products.