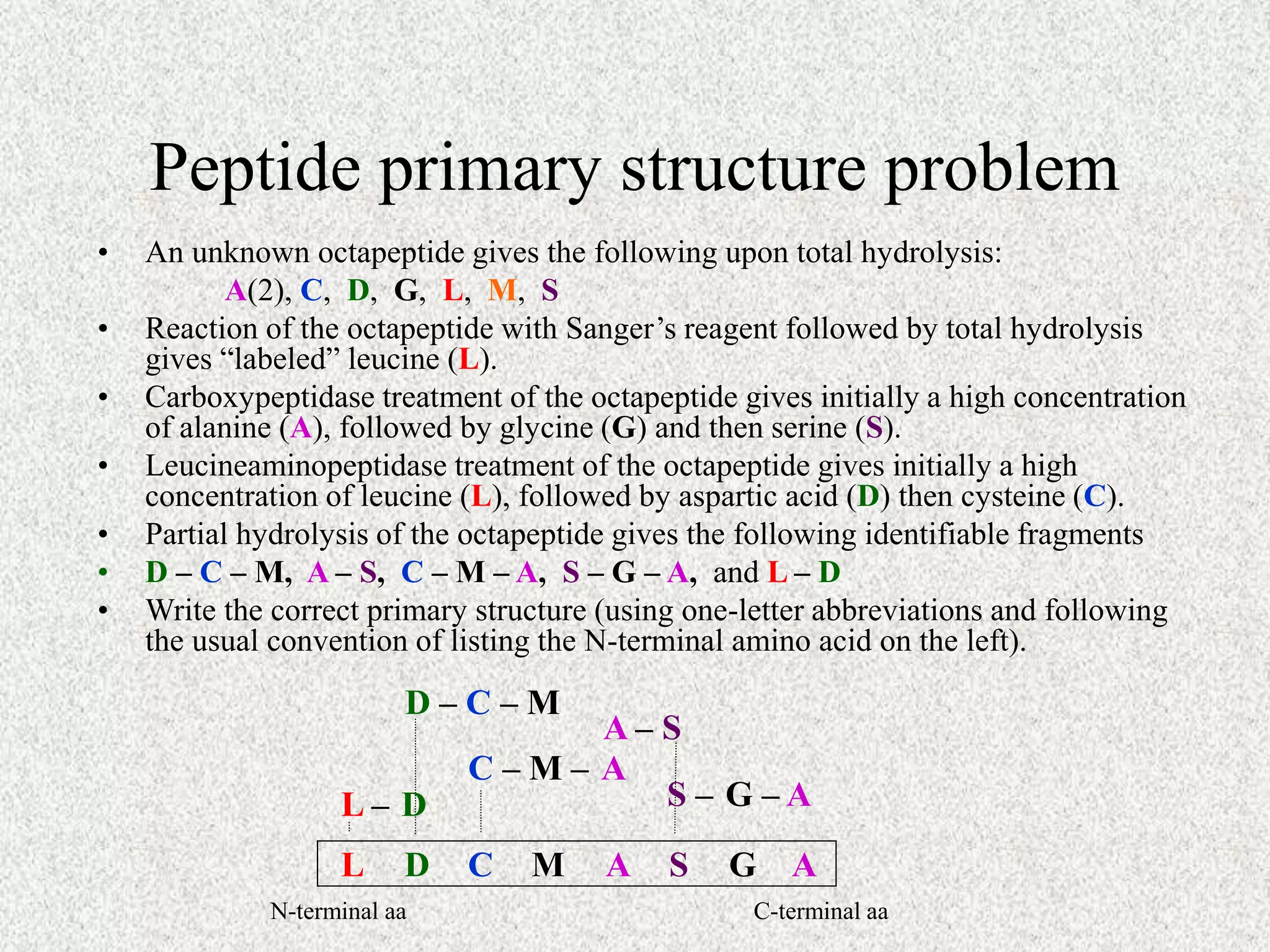
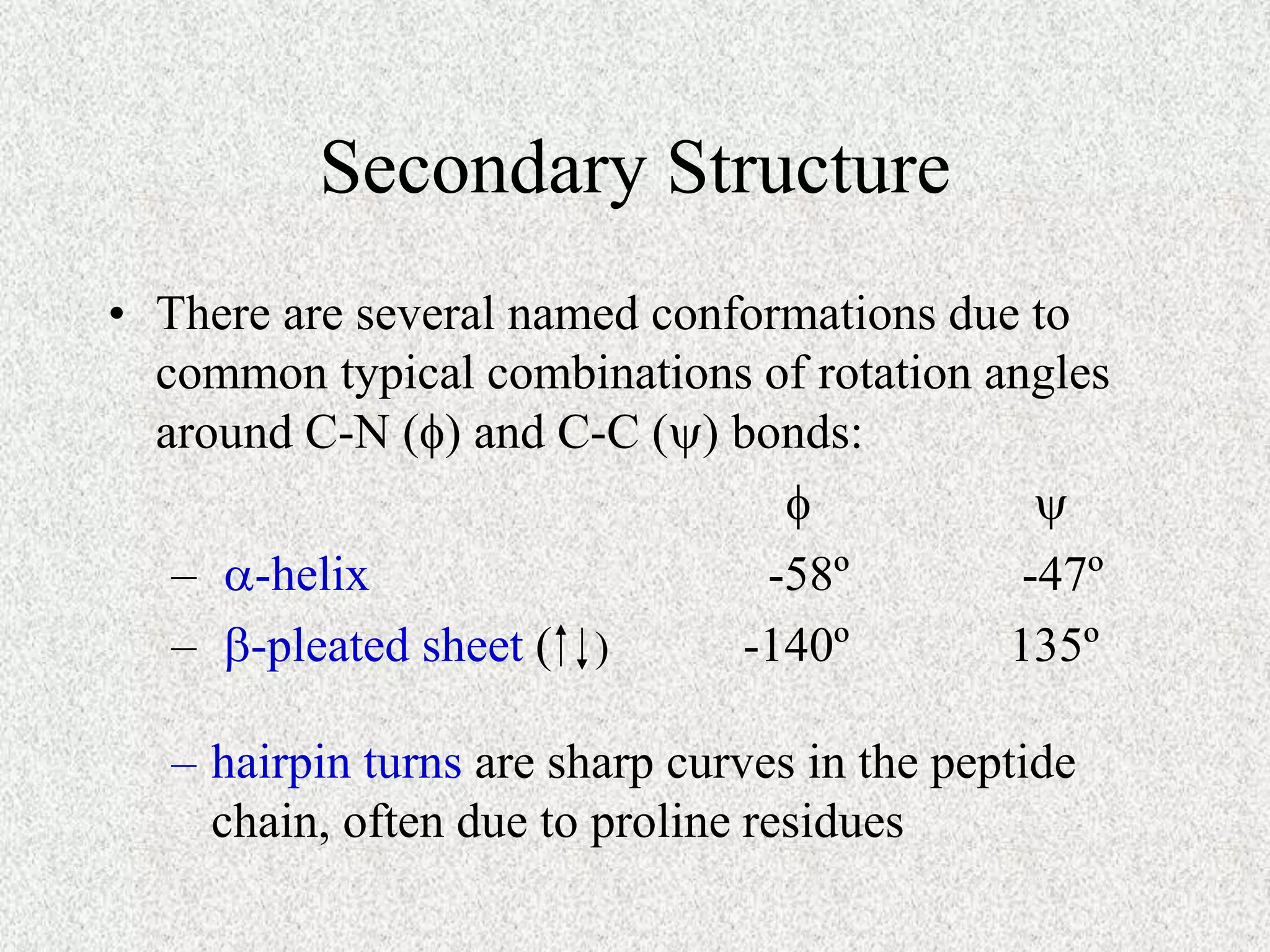
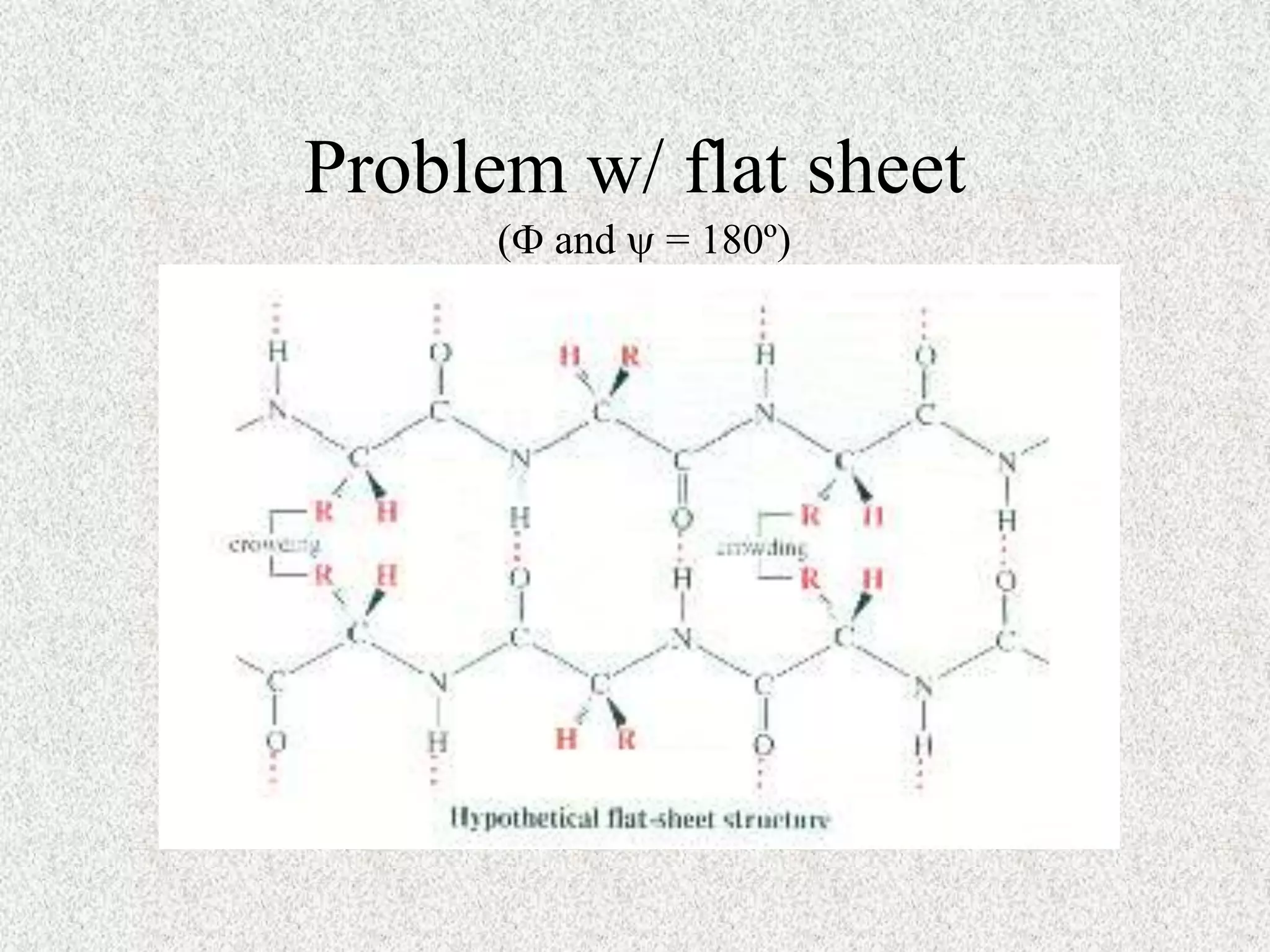
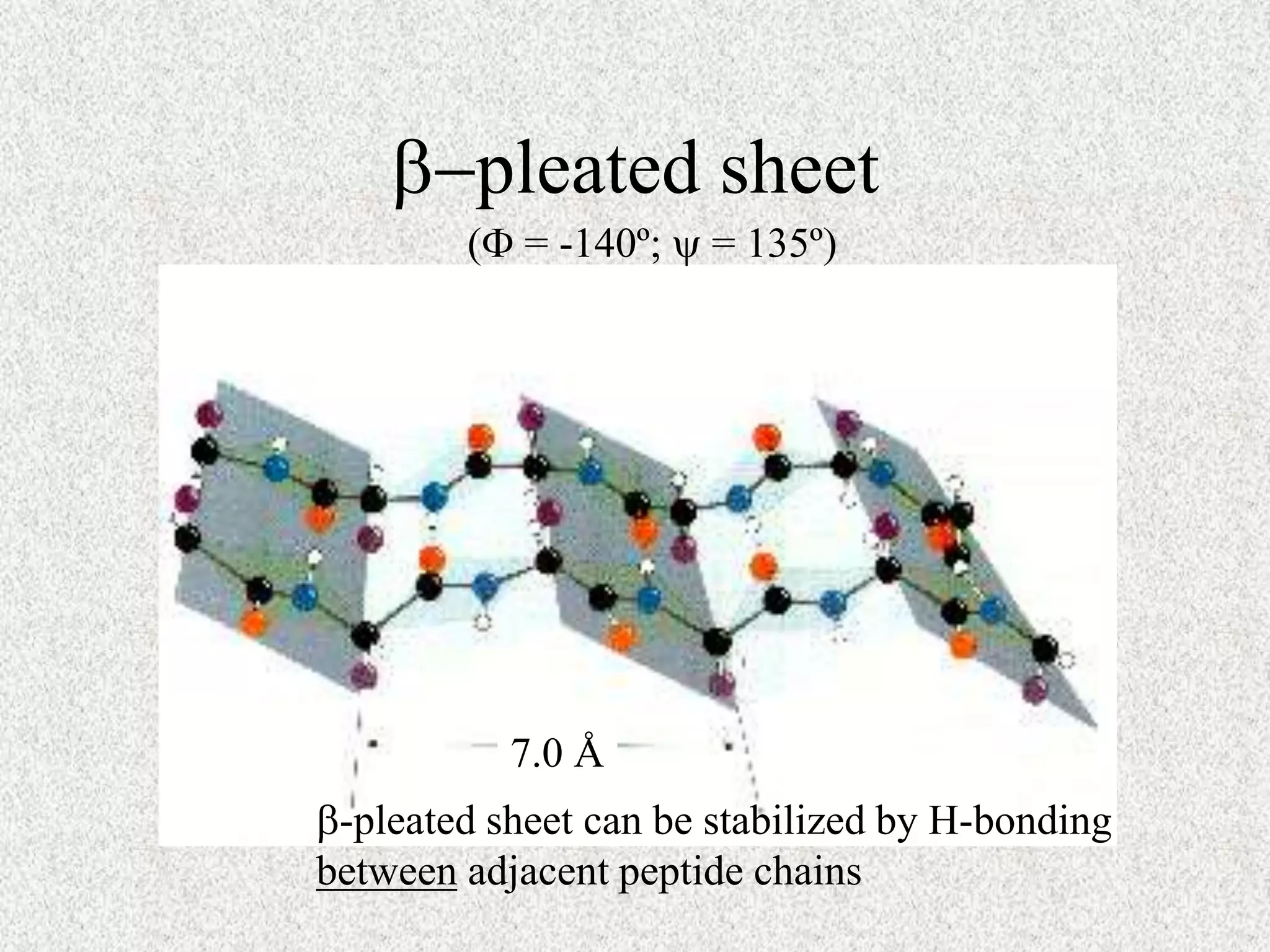
The octapeptide contains the amino acids A, C, D, G, L, M, S. Enzyme digestion and mass spectrometry identify the fragments D-C-M, A-S, C-M-A, S-G-A, and L-D. This information determines the primary structure is L-A-G-S-D-C-M-A. Secondary structure is based on bond rotations forming elements like alpha helices and beta pleated sheets. Tertiary structure describes the overall shape from peptide chain folding while quaternary structure involves interactions of multiple protein subunits.