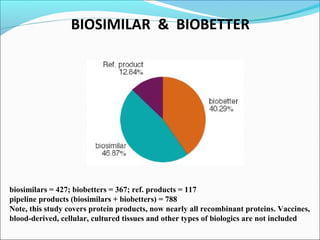
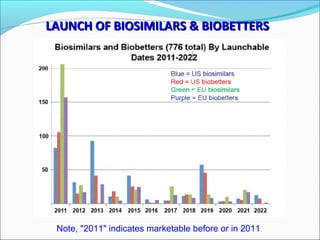
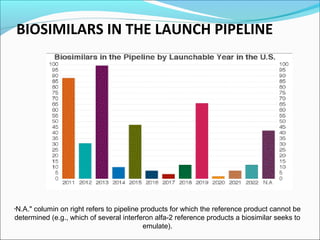
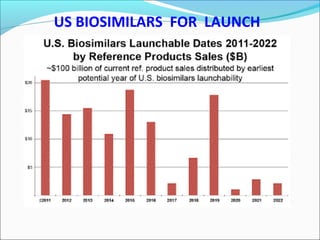
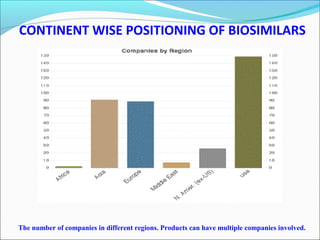
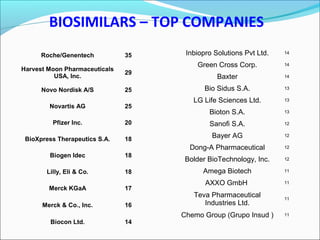
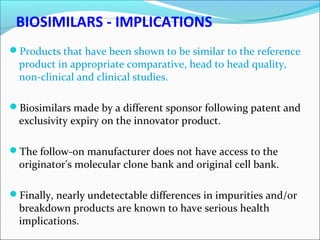
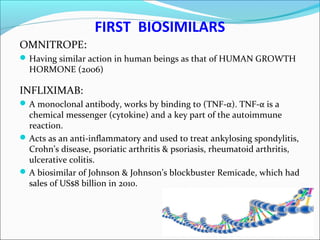
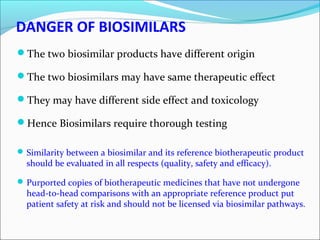
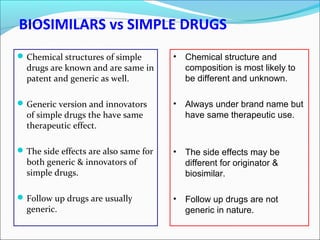
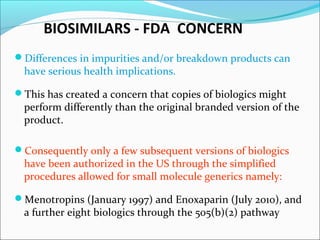
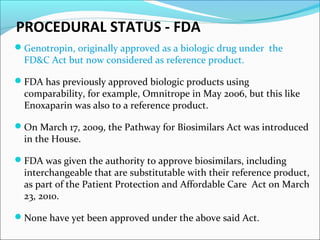
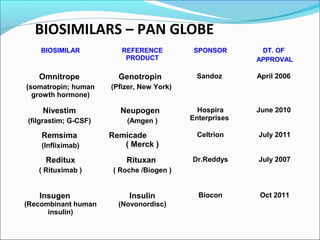
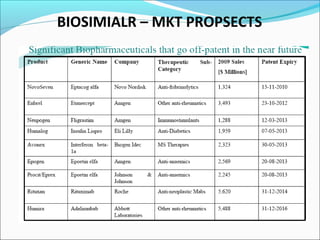
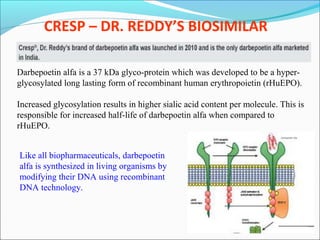
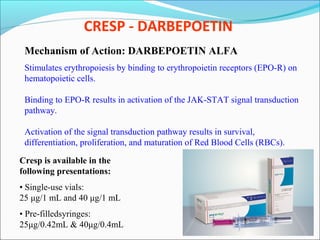
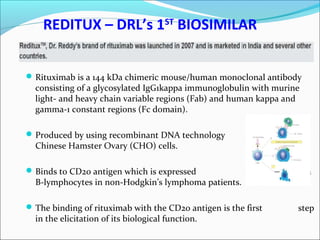
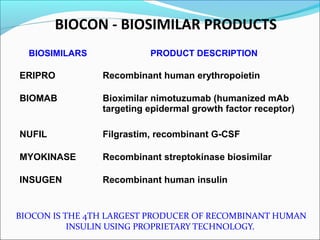
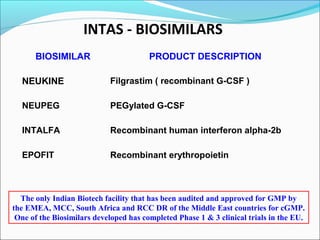
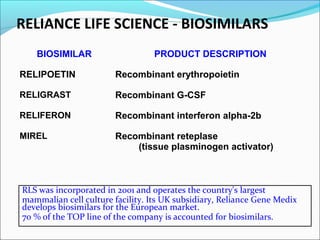
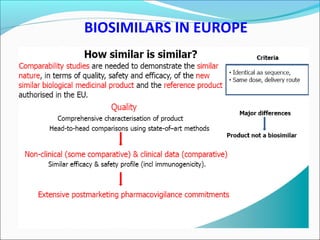
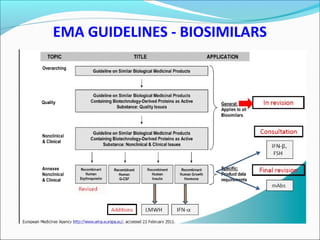
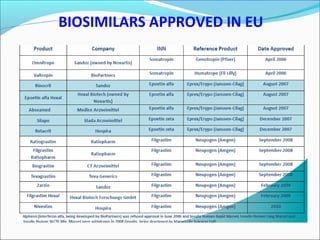
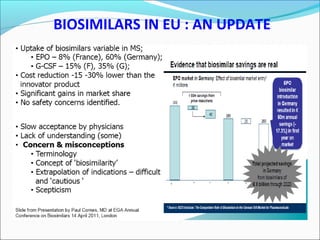
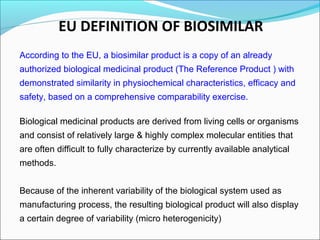
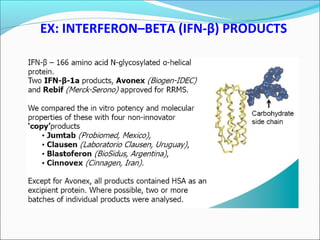
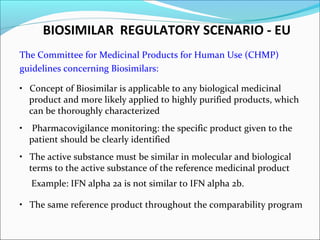
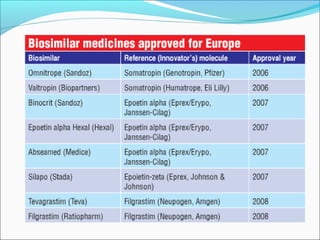
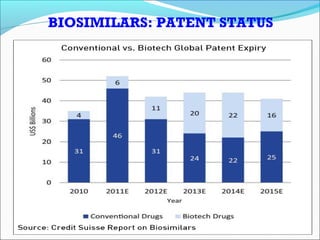
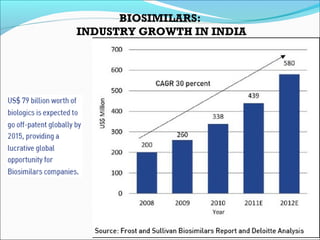
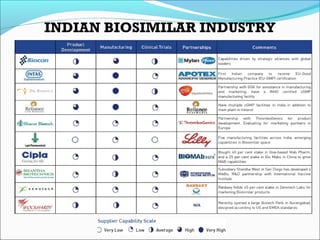
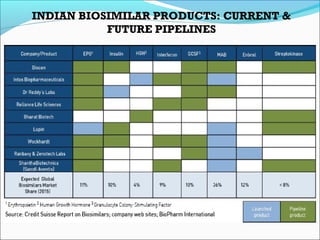
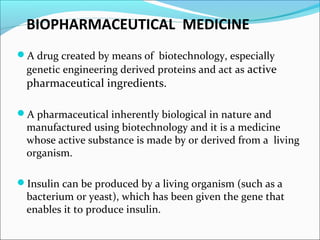
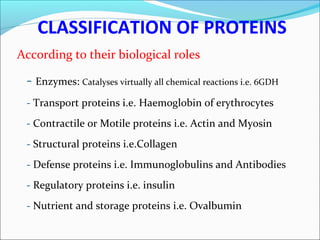
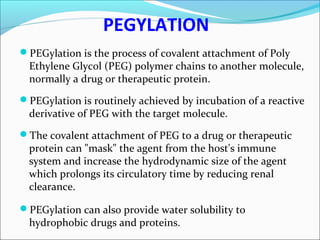
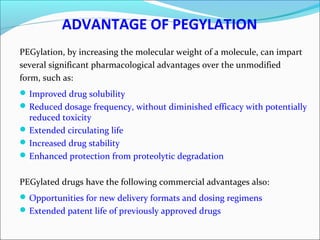
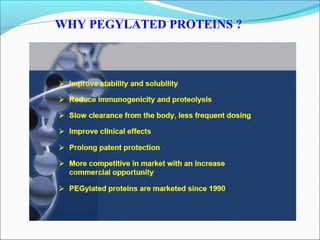
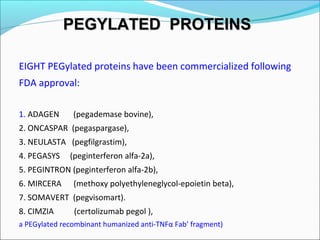
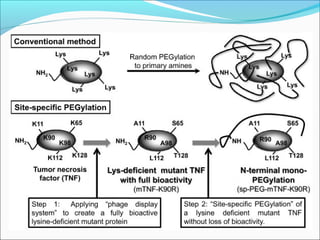
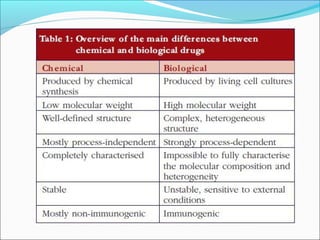
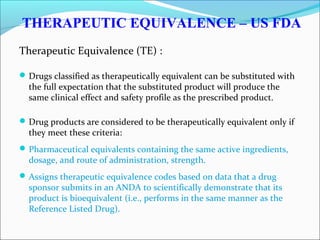
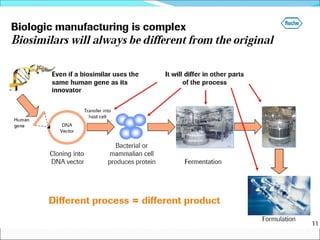
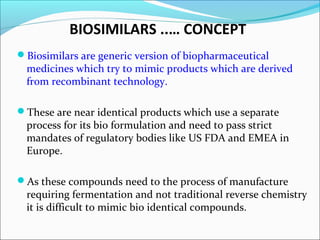
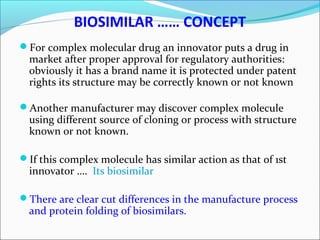
The document defines drugs and devices under the Drugs and Cosmetics Act of 1940 in India. It states that drugs include all medicines, substances and components for internal or external use, while devices are meant to treat, mitigate or prevent disease. Biologics and biosimilars are also included. The document then provides definitions and explanations of biotechnology, biopharmaceuticals, proteins drugs, pegylation, biologics vs biosimilars, and the FDA approach to biosimilars.







![BIOLOGICS vs BIOSIMILARS
Even though the FDA has been establishing standards for
licensure to ensure the safety and effectiveness of biosimilars
and issued a guidance in February 2012 [7], the FDA has not
approved a biological product as biosimilar or
interchangeable.
Several companies are developing biosimilars and will almost
certainly submit applications for licensure under the new law.
It is not yet known when the first biosimilar product will be
on the U.S. market.](https://image.slidesharecdn.com/pegylationbiosimilars-globalscenario-130813062033-phpapp02/85/Pegylation-biosimilars-global-scenario-25-320.jpg)