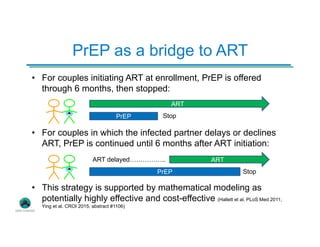
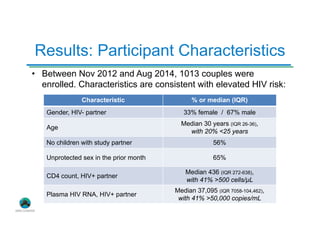
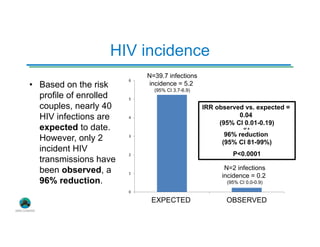
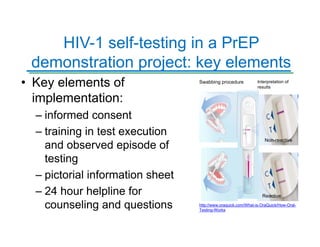
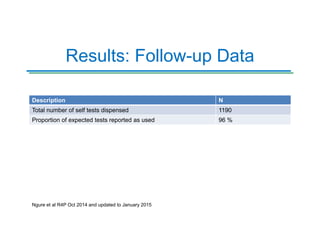
The Partners Demonstration Project is studying the use of antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) to prevent HIV among heterosexual couples in Kenya and Uganda. An ancillary study is evaluating the acceptability of HIV self-testing among individuals using PrEP in Kenya. Preliminary findings show high uptake of self-testing, with 96% of expected tests reported as used. Qualitative feedback indicates self-testing reduces anxiety and empowers individuals. Continued research will provide more data on experiences with self-testing and its potential as a cost-effective component of PrEP programs.










![Results: Qualitative
• Quotes from 4 focus group discussions conducted so far:
– Reduced anxiety
• “Every day, every time thinking to yourself, “How will it be when I go back there
[clinic]? When you test yourself, you know your status, you relax”
– Testing alone versus with others:
• “Sometimes I call my husband. Like the last time I tested, I called my husband
and told him “Come you see mine is okay until now “.
• “Like me, I hide myself… (All the participants laugh) I go to the bedroom.”
– Remembering to test:
• “I put a reminder on my phone.”
• “What makes me remember is putting it together with these medication
(Truvada).”](https://image.slidesharecdn.com/partnersdemonstrationproject-hivselftestingupdate-feb2015-150330203318-conversion-gate01-150601170859-lva1-app6891/85/Partners-Demonstration-Project-HIV-self-testing-update-Feb-2015-16-320.jpg)


