This document discusses the development and use of a rapid, non-invasive at-home HIV self-test called OraQuick. It describes:
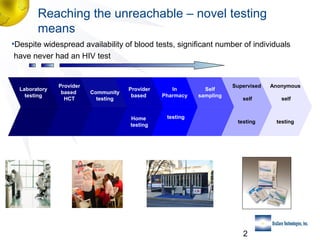
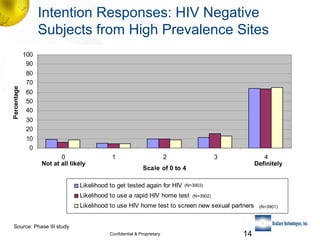
1) The need for novel testing approaches to reach more individuals who have never been tested for HIV.
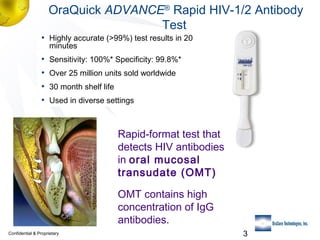
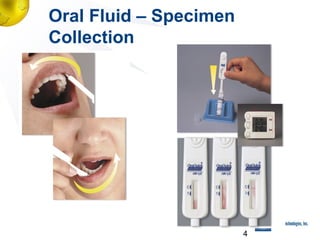
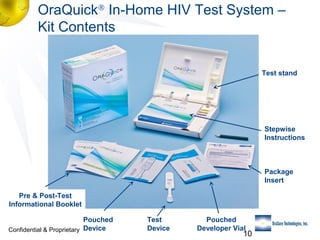
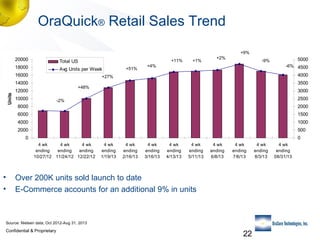
2) Details of the OraQuick test, including its accuracy (>99%), ease of use involving a oral fluid sample, and 30 month shelf life.
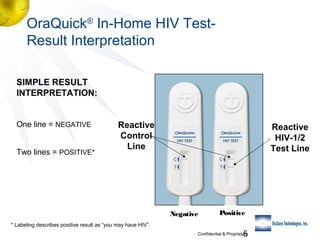
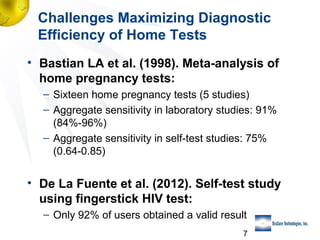
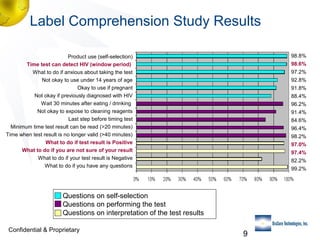
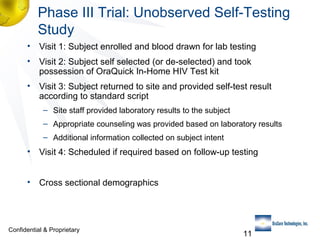
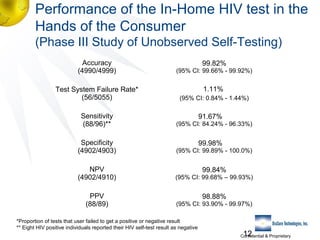
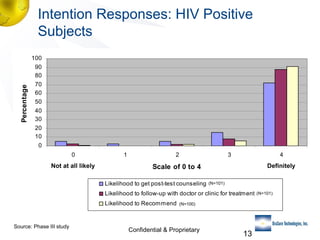
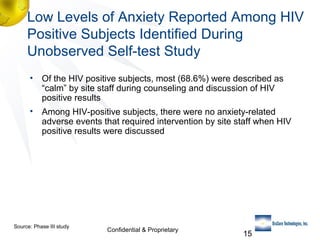
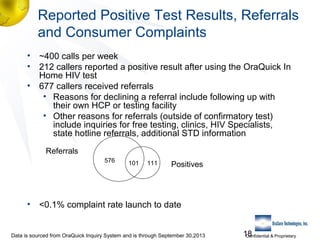
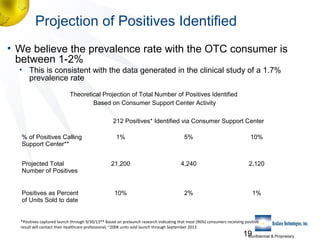
3) Clinical trial results showing the test had a 99.8% accuracy rate when used unsupervised and identified previously undiagnosed HIV-positive individuals, though some reported incorrect negative results.
4) Support resources like a 24/7 support center to help individuals interpret results and get linked to care