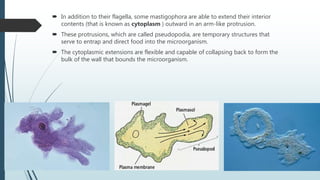
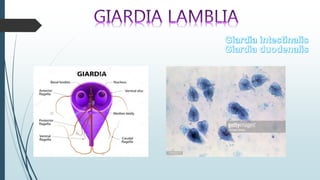
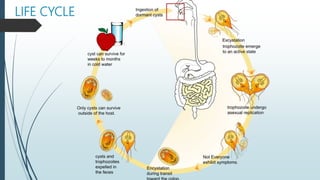
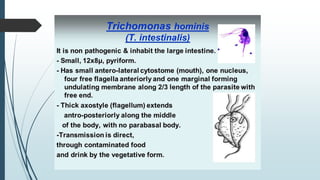
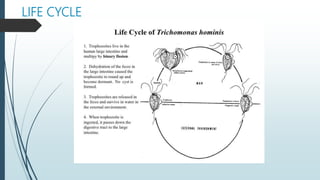
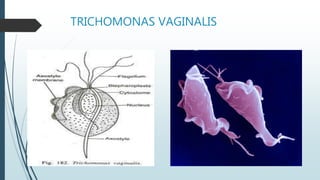
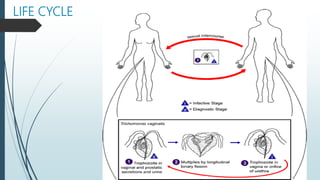
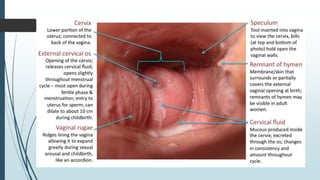
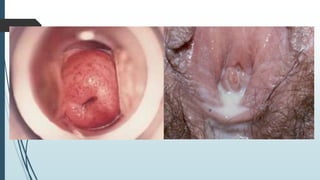
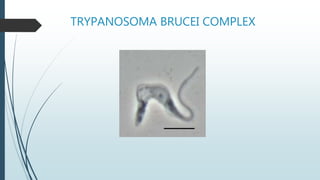
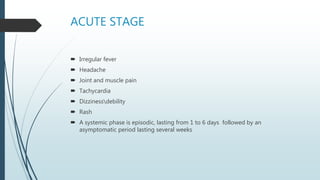
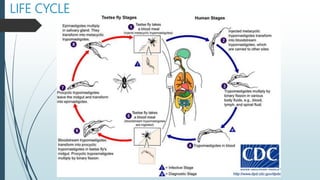
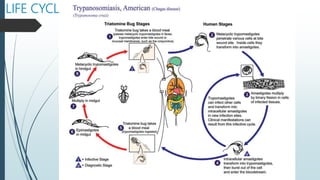
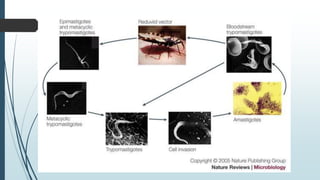
Mastigophora is a division of single-celled protozoans, characterized by species that possess flagella for movement and can form pseudopodia for feeding. Giardia lamblia, a notable member, is a parasite that causes giardiasis, primarily transmitted through contaminated water and leading to various gastrointestinal symptoms. Other protozoans like Trichomonas vaginalis and Trypanosoma brucei can cause significant infections such as trichomoniasis and African sleeping sickness, with distinct life cycles, modes of transmission, and clinical impacts.