Embed presentation
Download to read offline
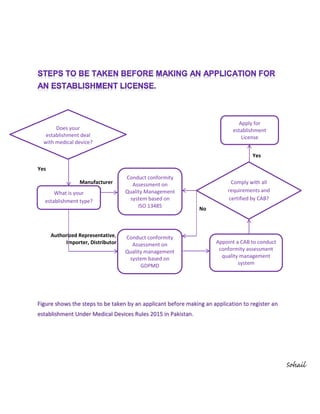

The document outlines the steps for an applicant to register an establishment under the medical devices rules of 2015 in Pakistan. It includes determining the establishment's involvement with medical devices, conducting conformity assessments based on relevant quality management standards, and applying for an establishment license. It emphasizes the need for compliance with requirements and certification by a competent authority.
