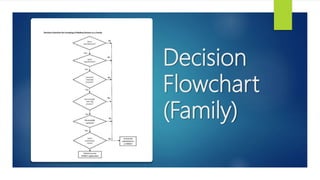
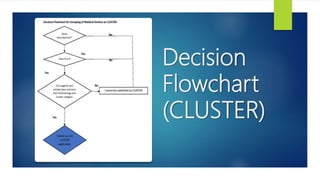
This document discusses the different categories that medical devices can be grouped into for listing in the Medical Device Register: single, family, system, set, IVD test kit, and IVD cluster. It provides examples and definitions for each category. The key rules for grouping are that devices must be from the same manufacturer, have a common intended purpose, and share proprietary name, risk classification, design, and manufacturing process. Flowcharts are also included to help determine which category a group of devices falls under.