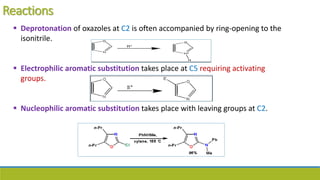
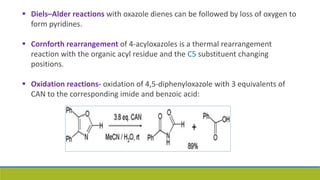
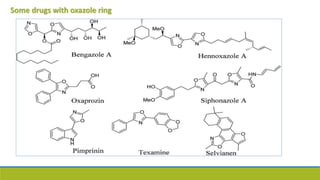
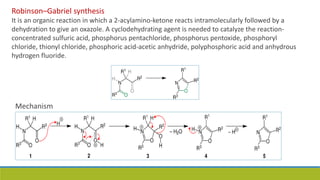
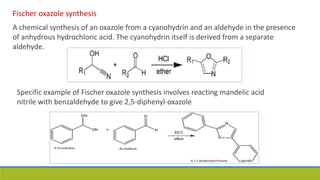
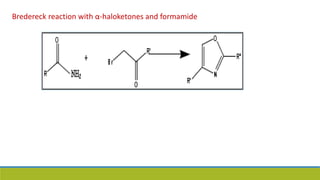
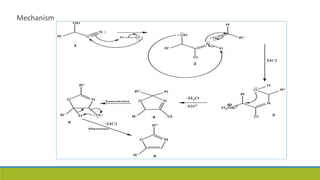
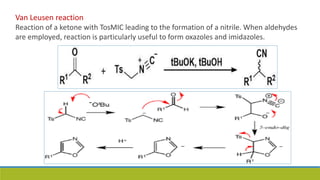
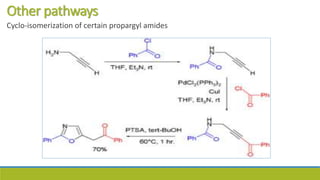
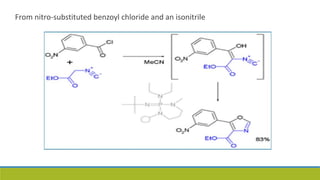
Oxazole is an aromatic compound containing an oxygen and nitrogen separated by one carbon. It is prepared through several syntheses including the Robinson-Gabriel synthesis using 2-acylaminoketones, Fischer oxazole synthesis from cyanohydrins and aldehydes, and the Bredereck reaction with α-haloketones and formamide. Oxazoles are weakly basic and are used in fungicides, pesticides, and various drugs due to their ability to undergo substitutions and reactions like Diels-Alder. They are also involved in the biosynthesis of non-ribosomal peptides from serine or threonine.



![Biosynthesis
Cyclization and oxidation of serine or threonine non-ribosomal peptides
Where X = H, CH3 for serine and threonine respectively, B = base
(1) Enzymatic cyclization (2) Elimination (3) [O] = enzymatic oxidation](https://image.slidesharecdn.com/oxazolevpnok-200411101922/85/Oxazole-11-320.jpg)