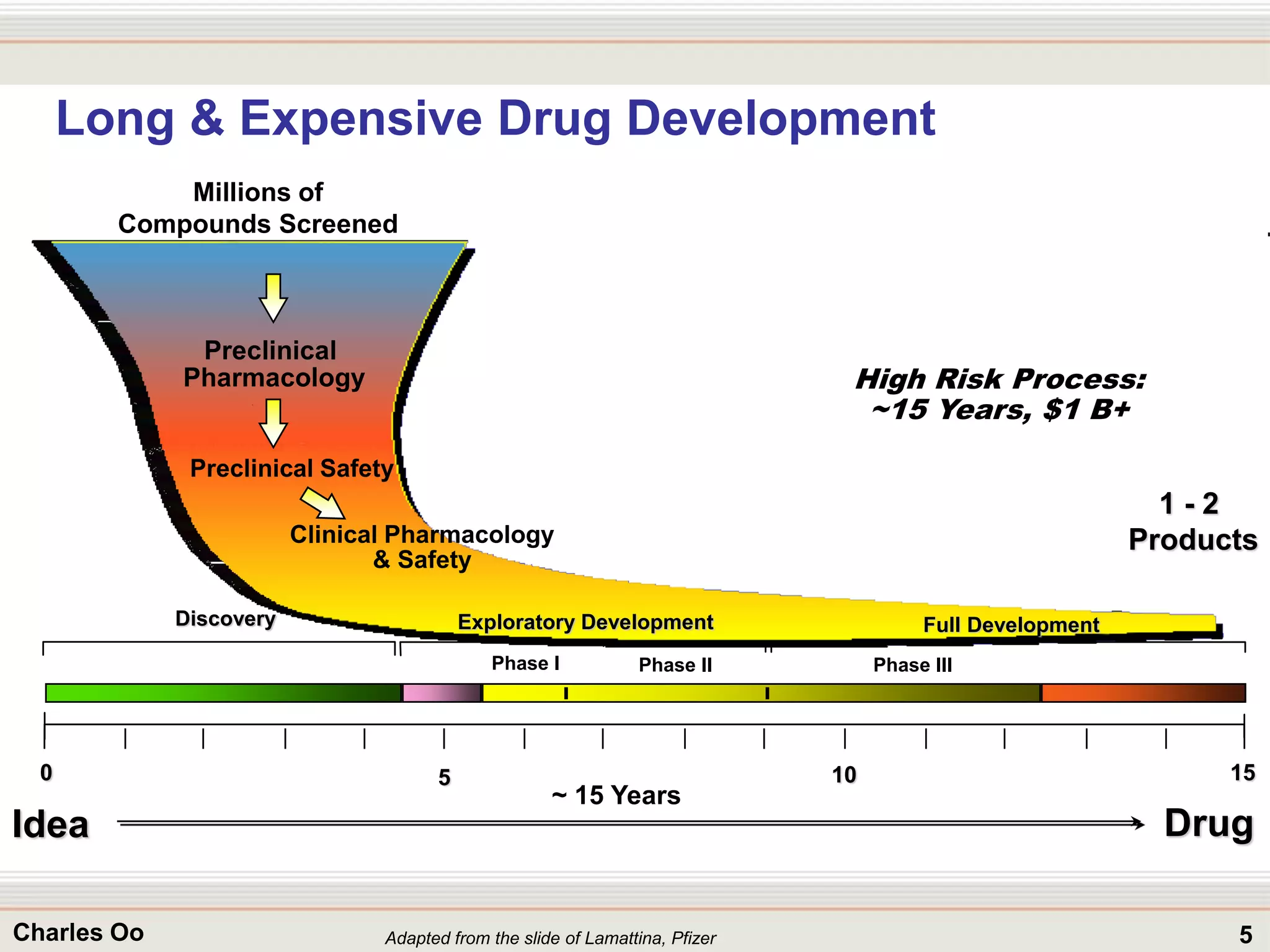
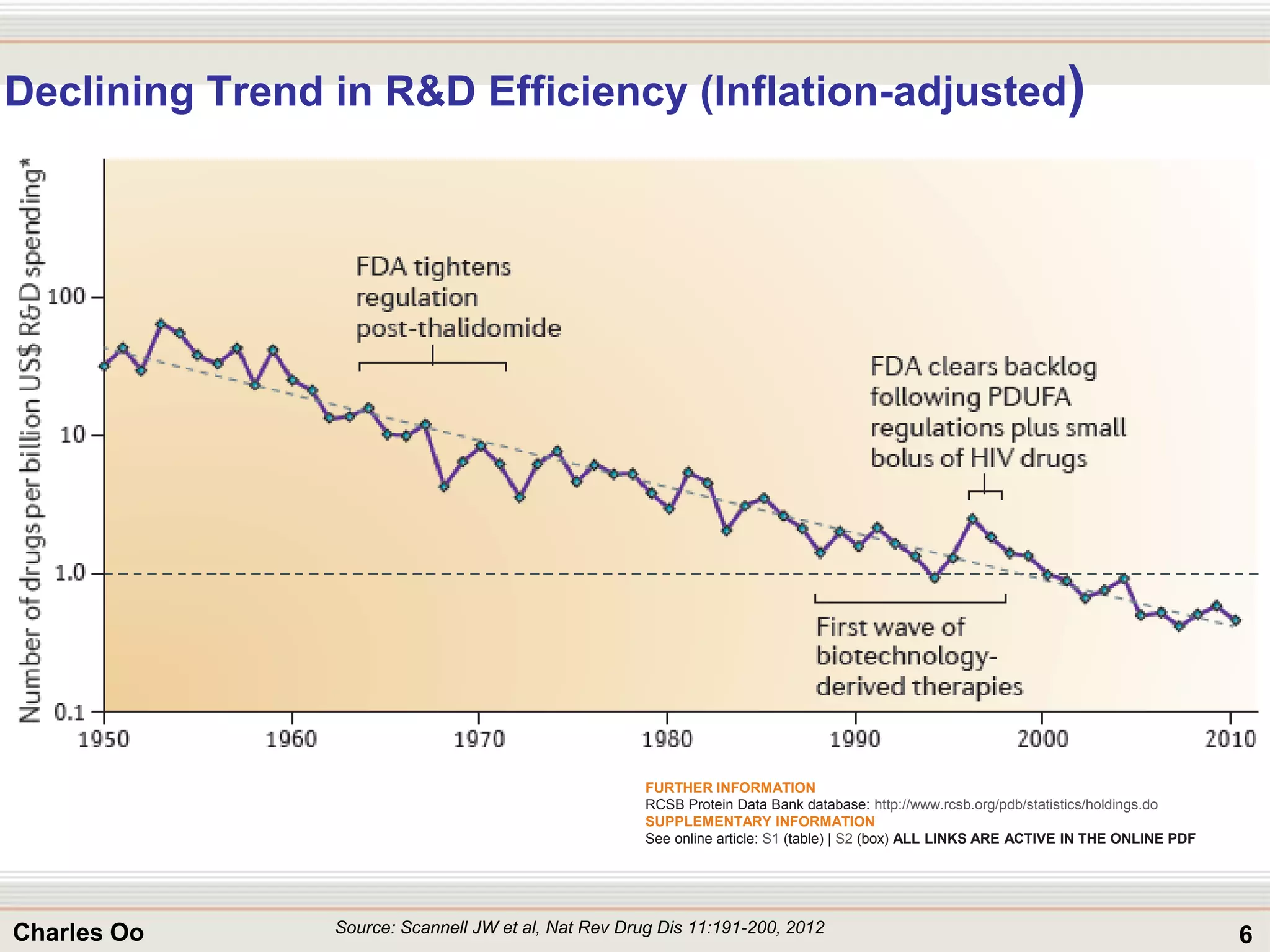
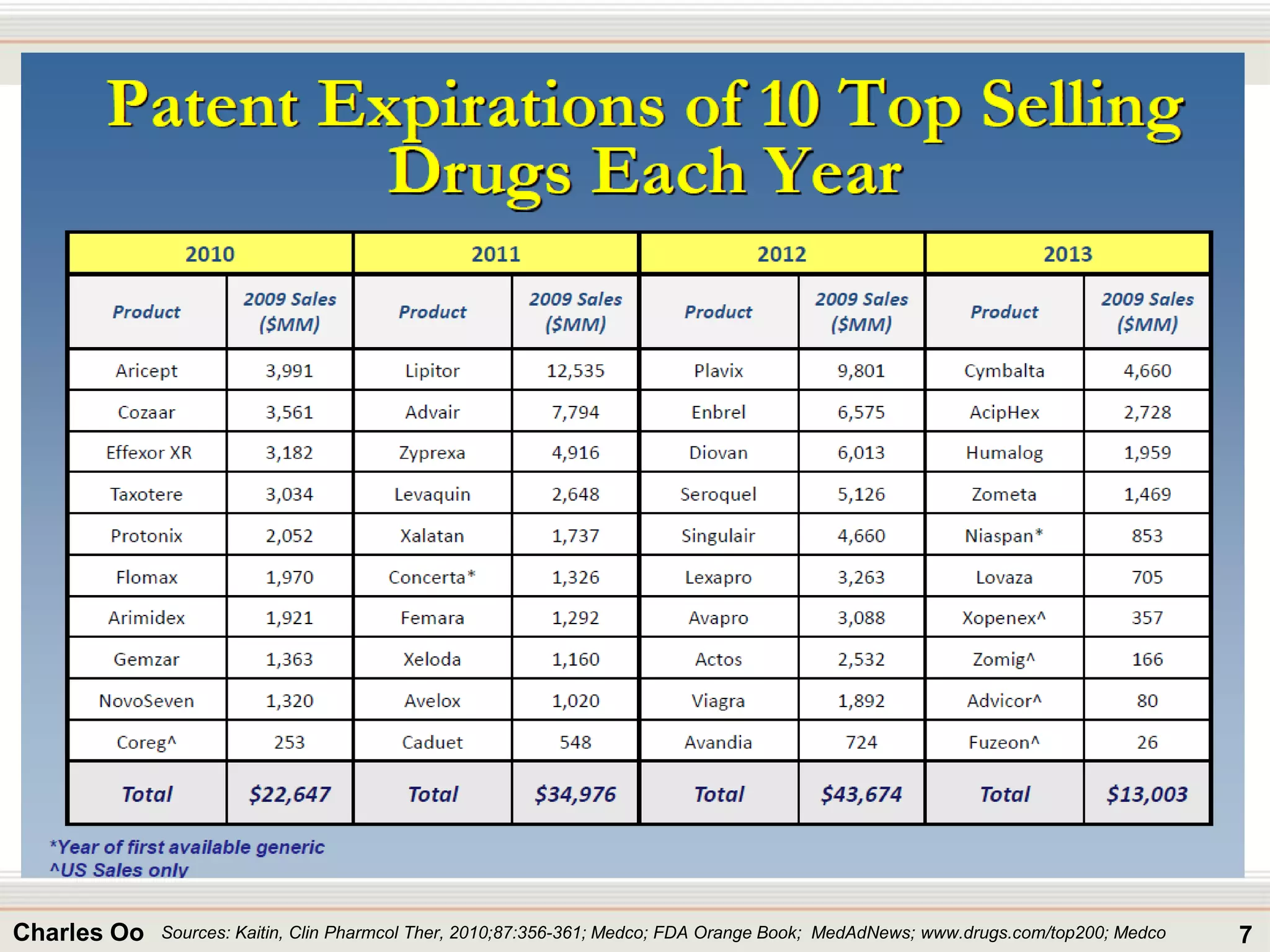
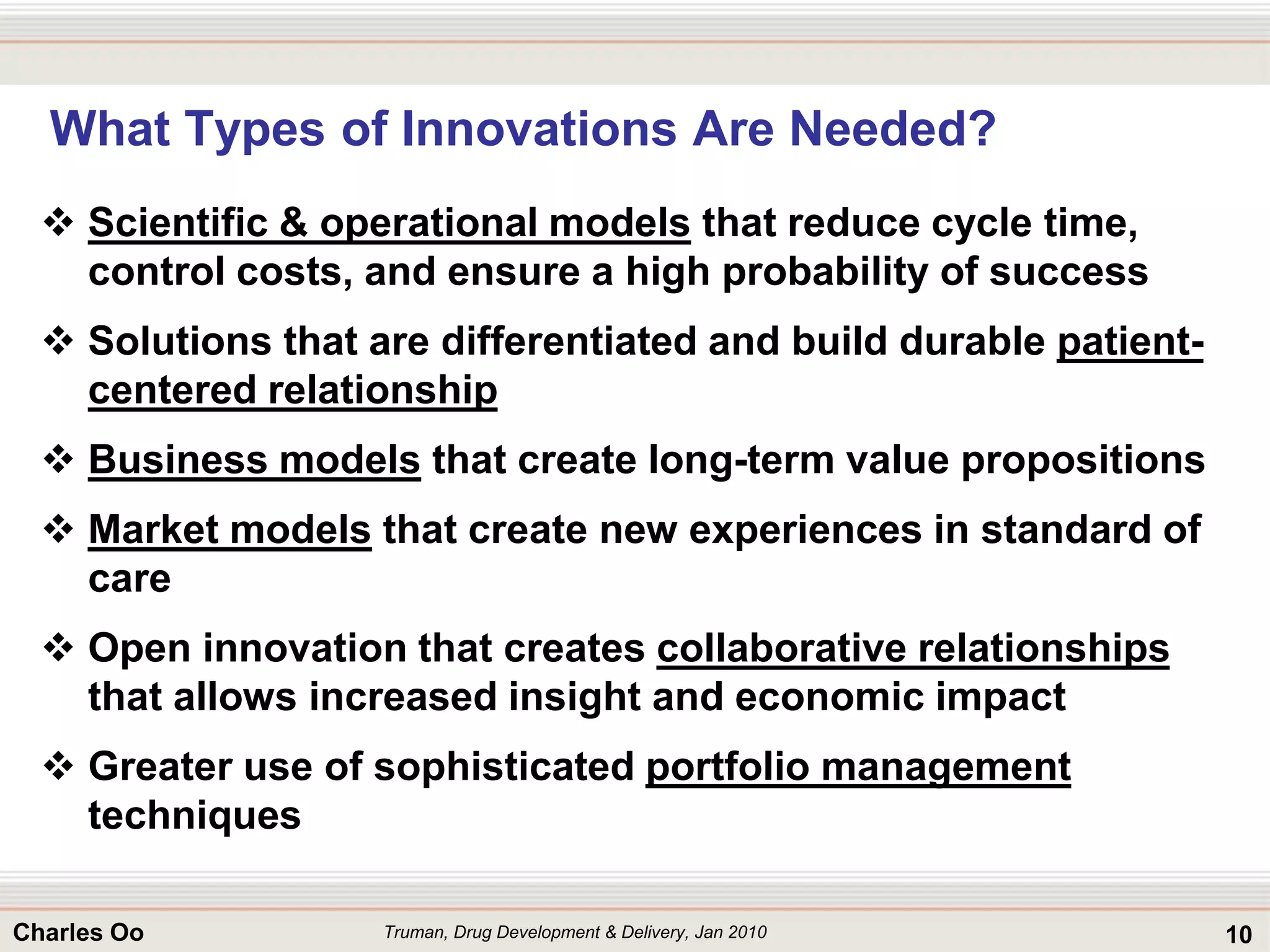
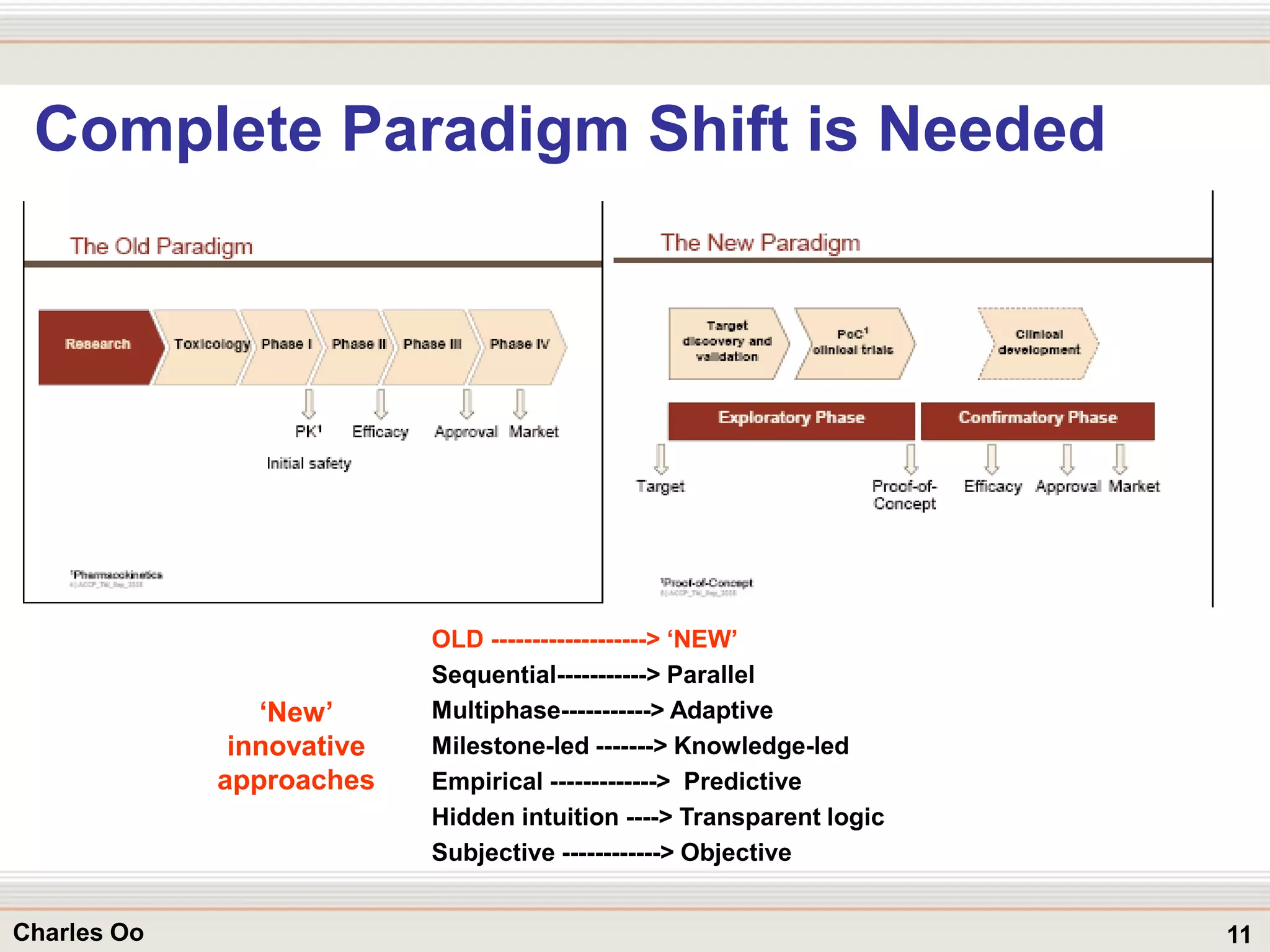
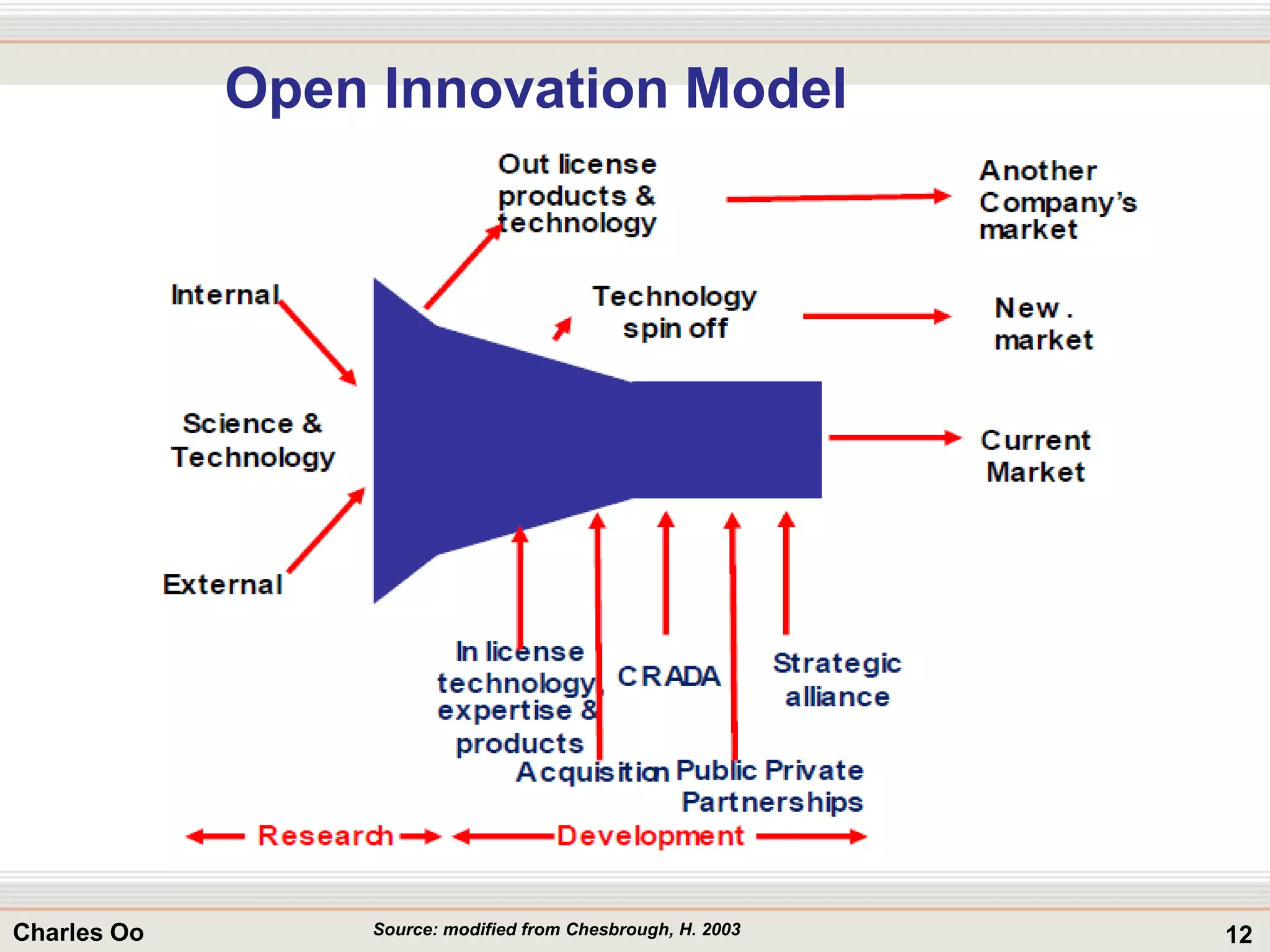
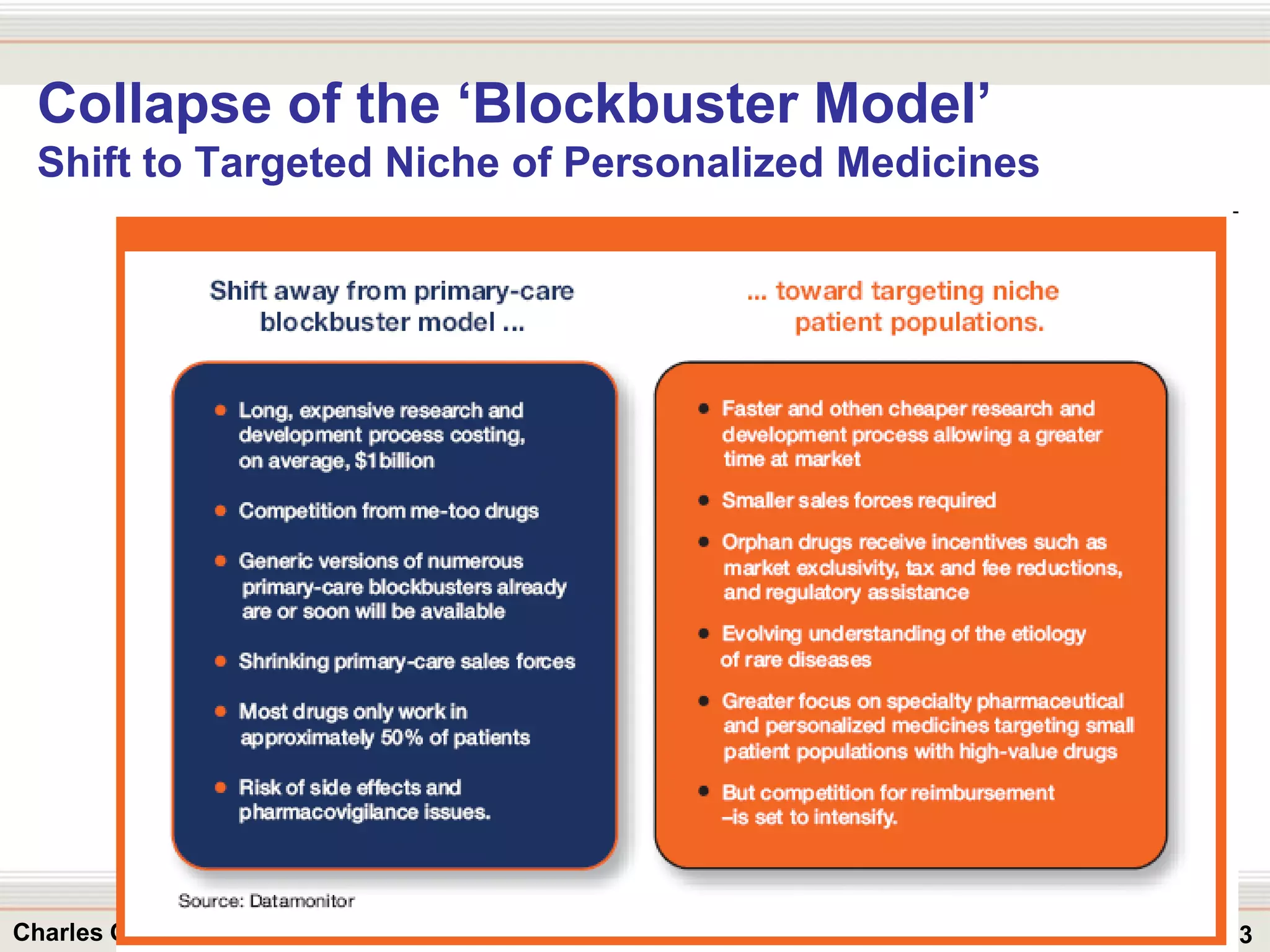
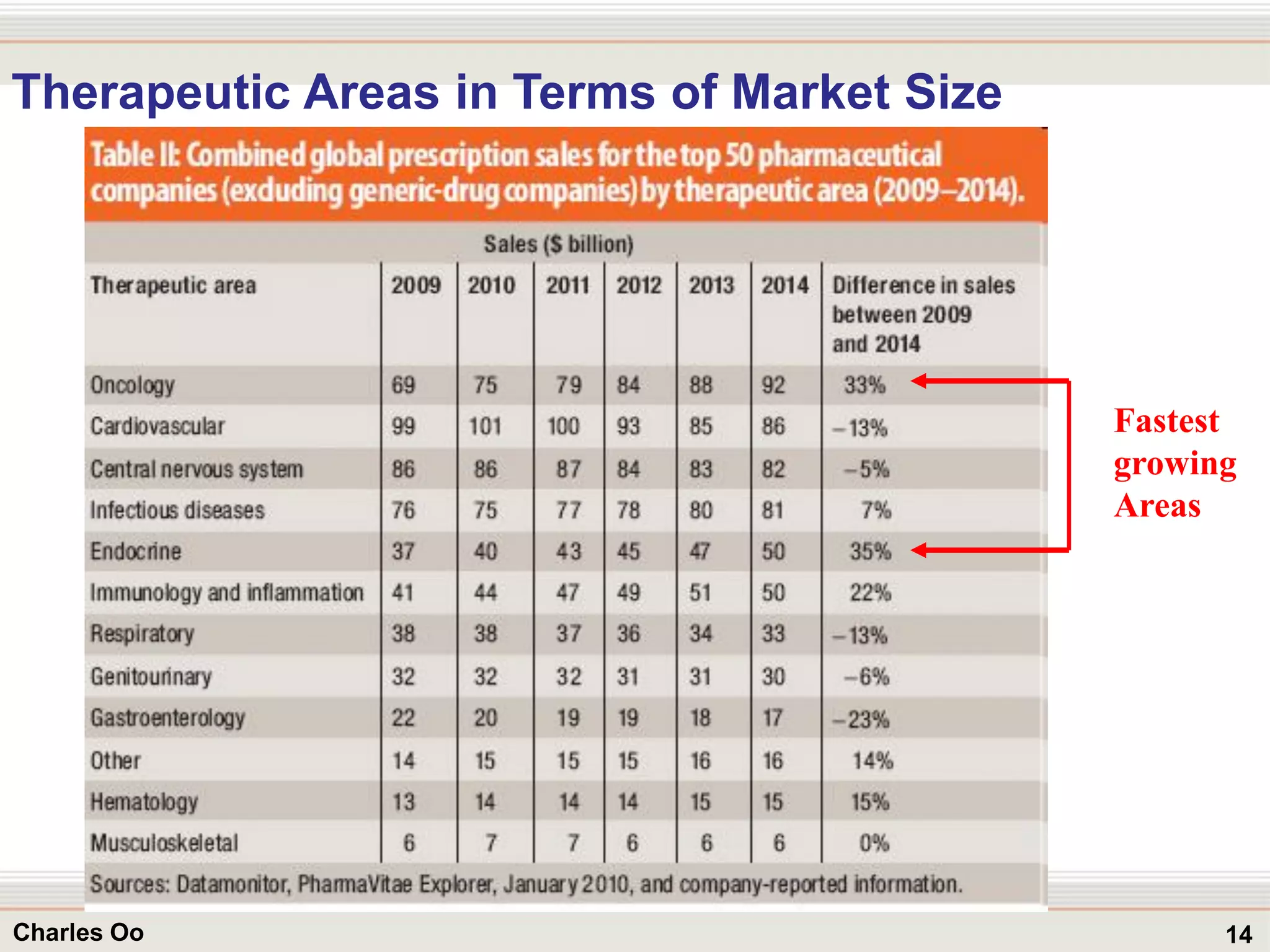
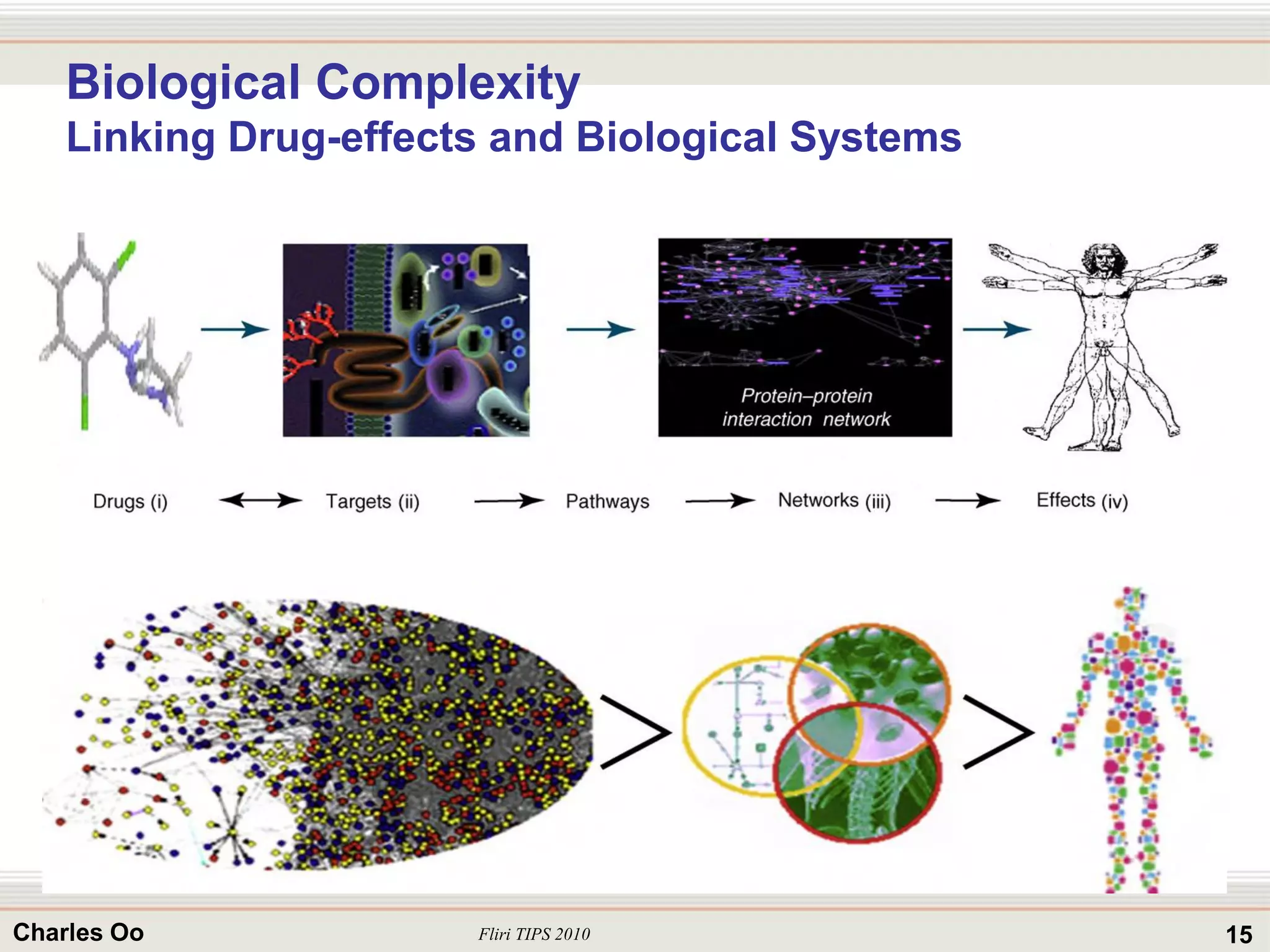
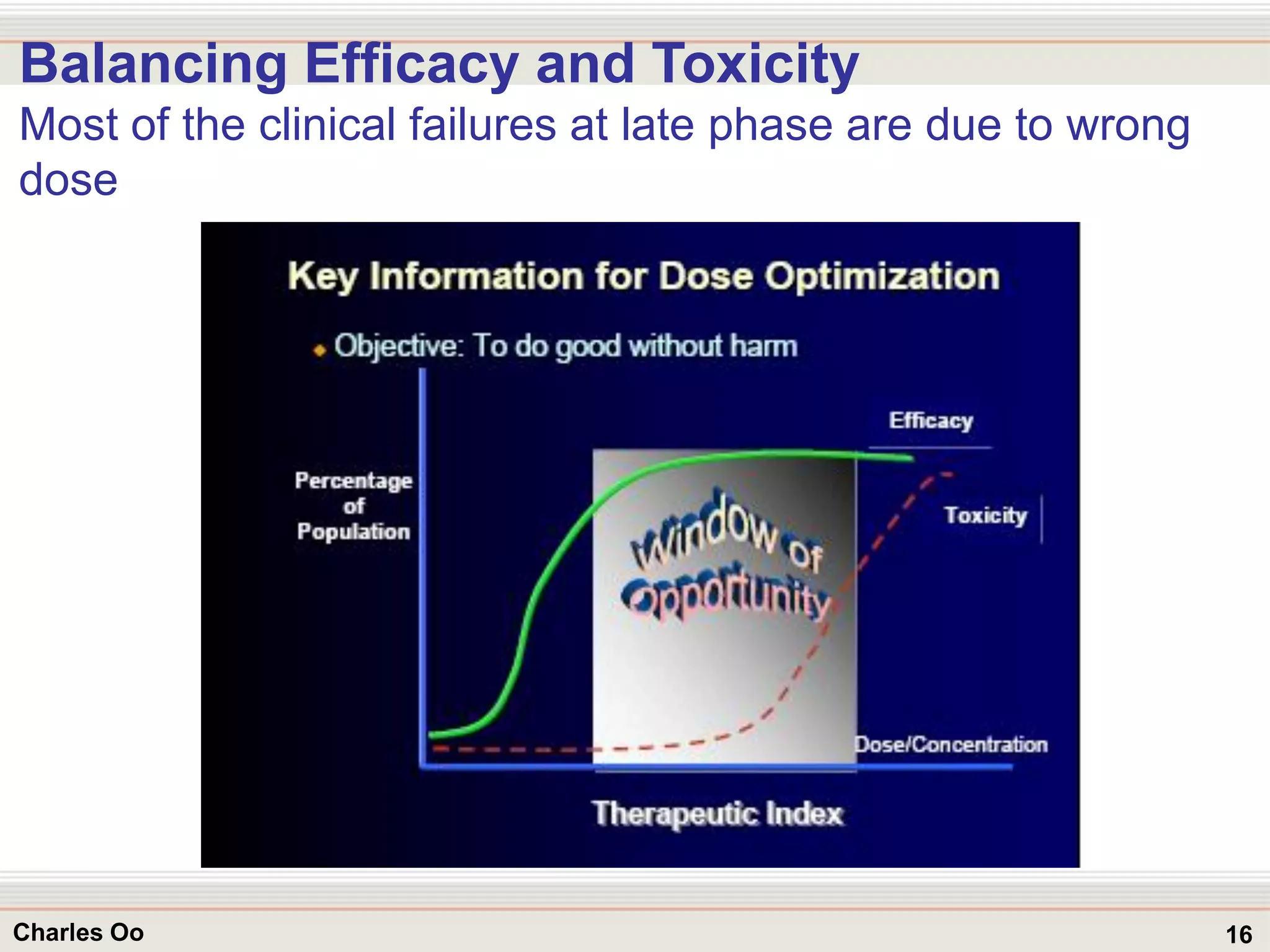
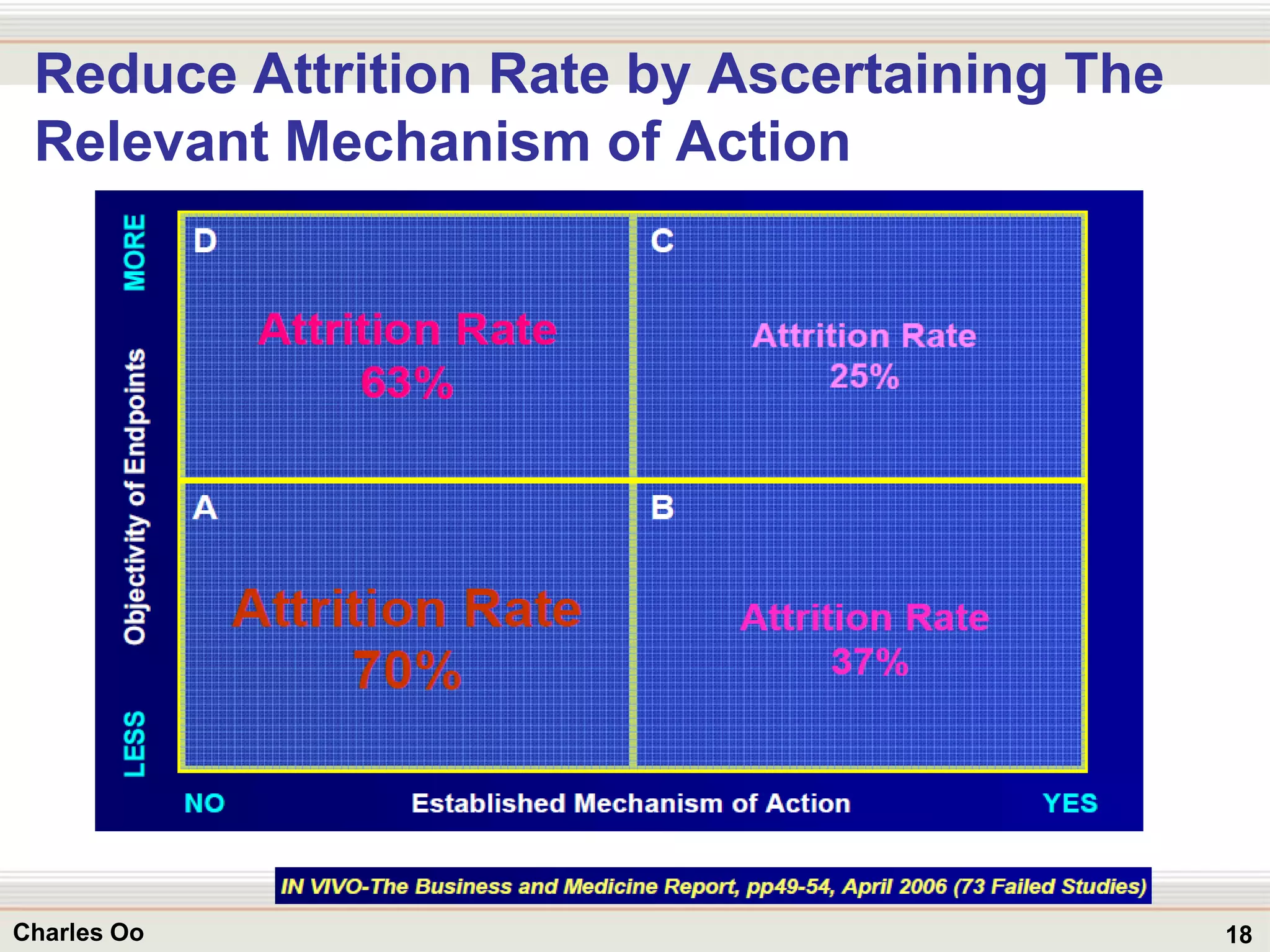
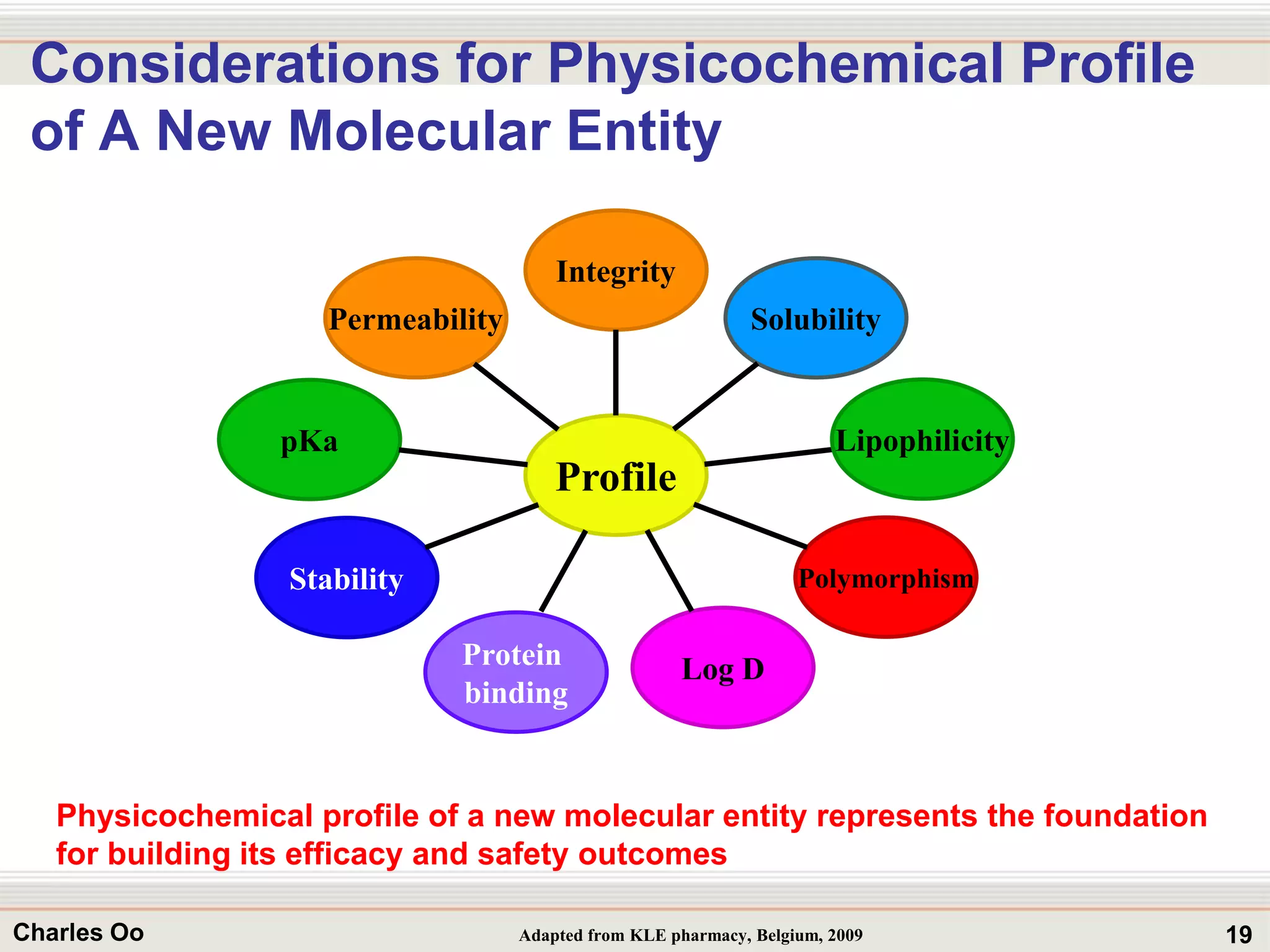
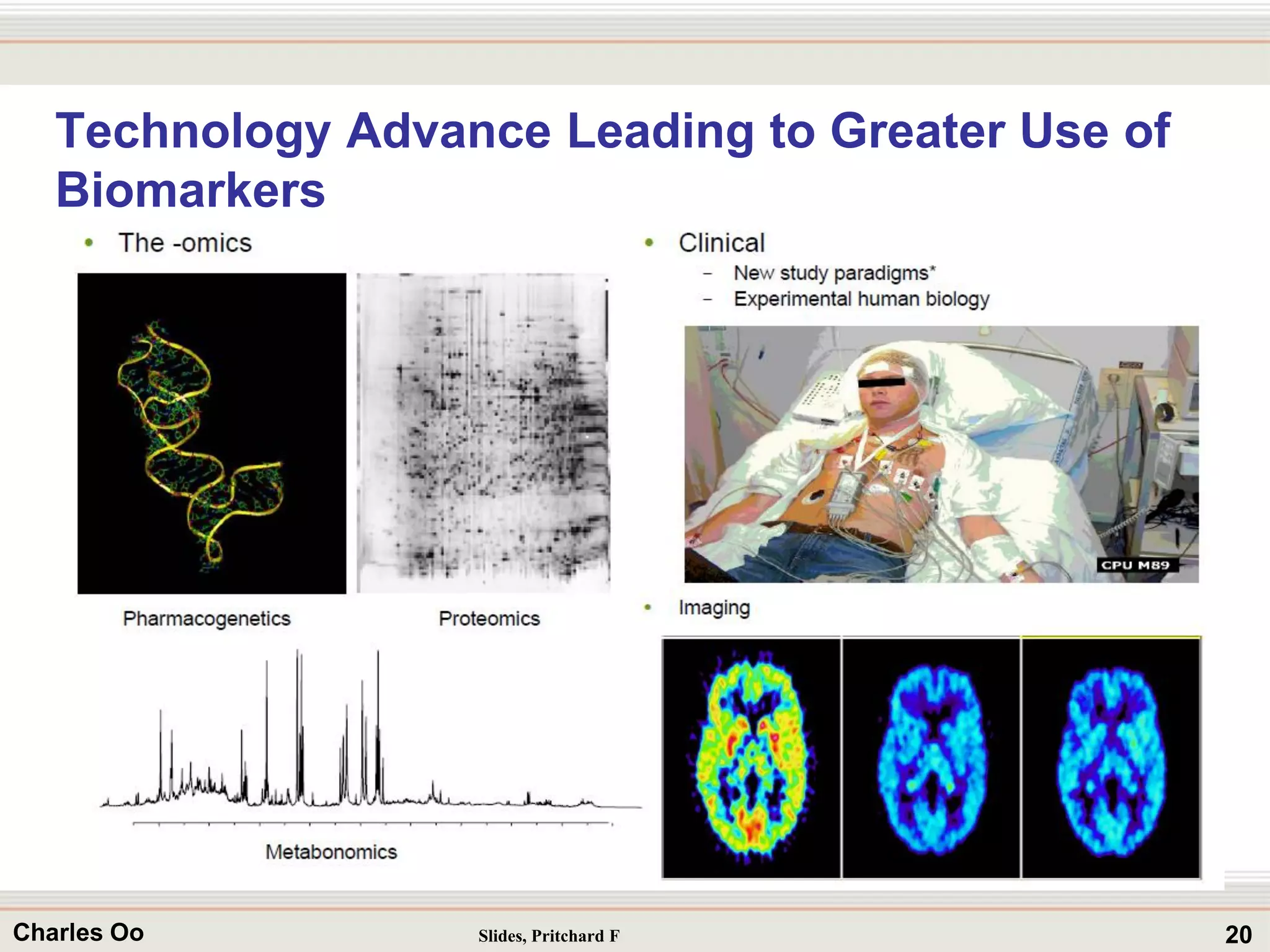
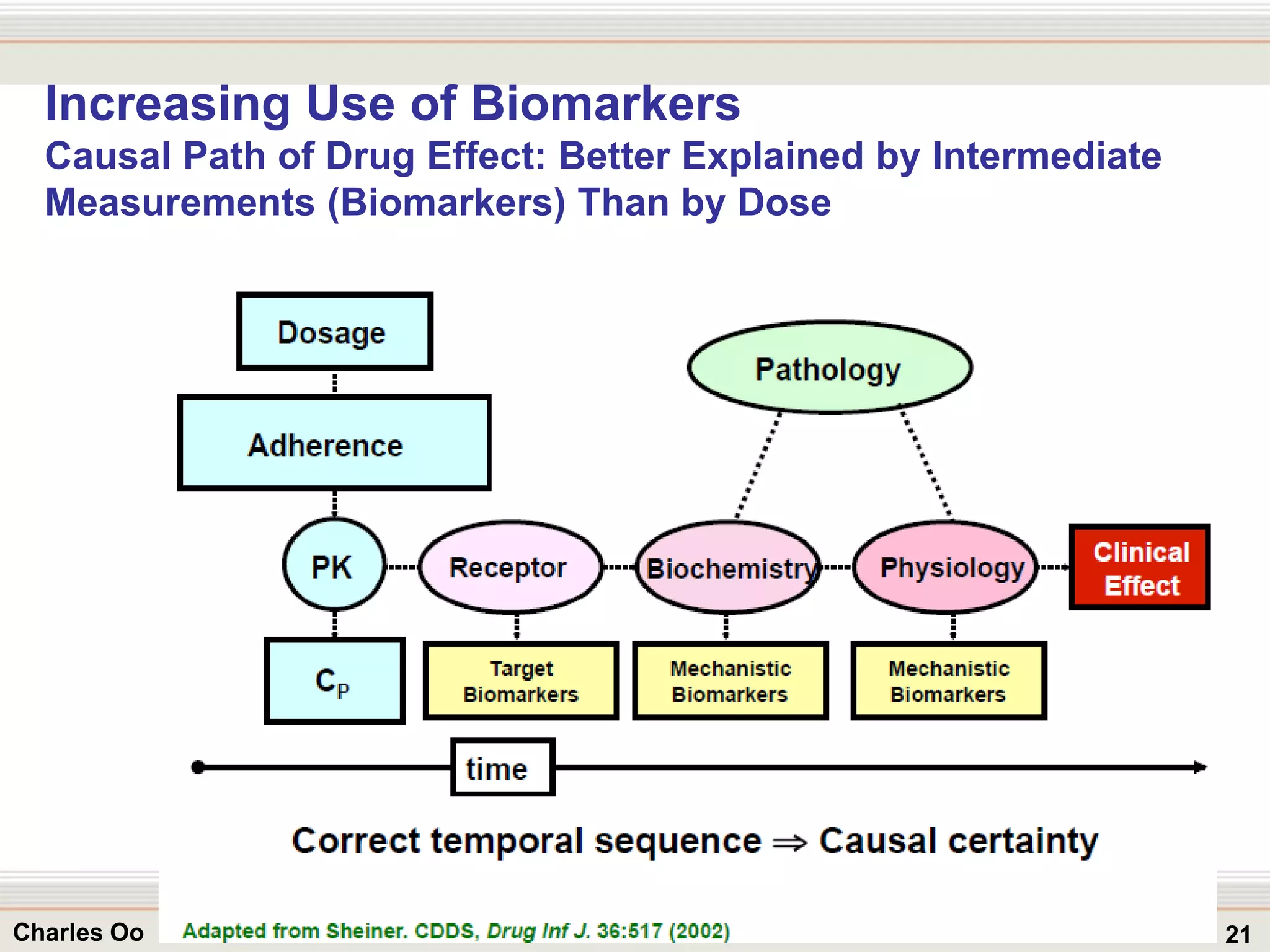
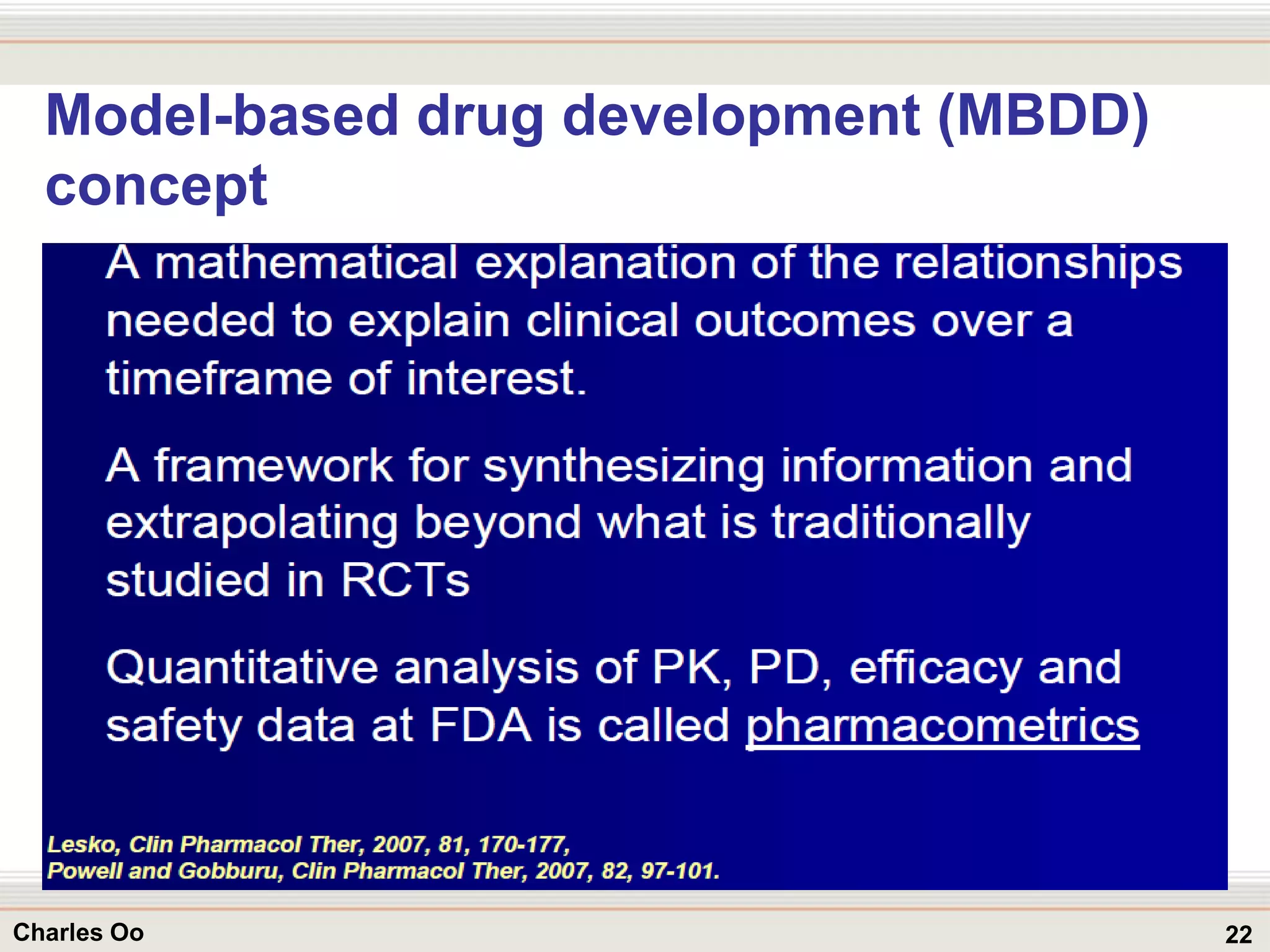
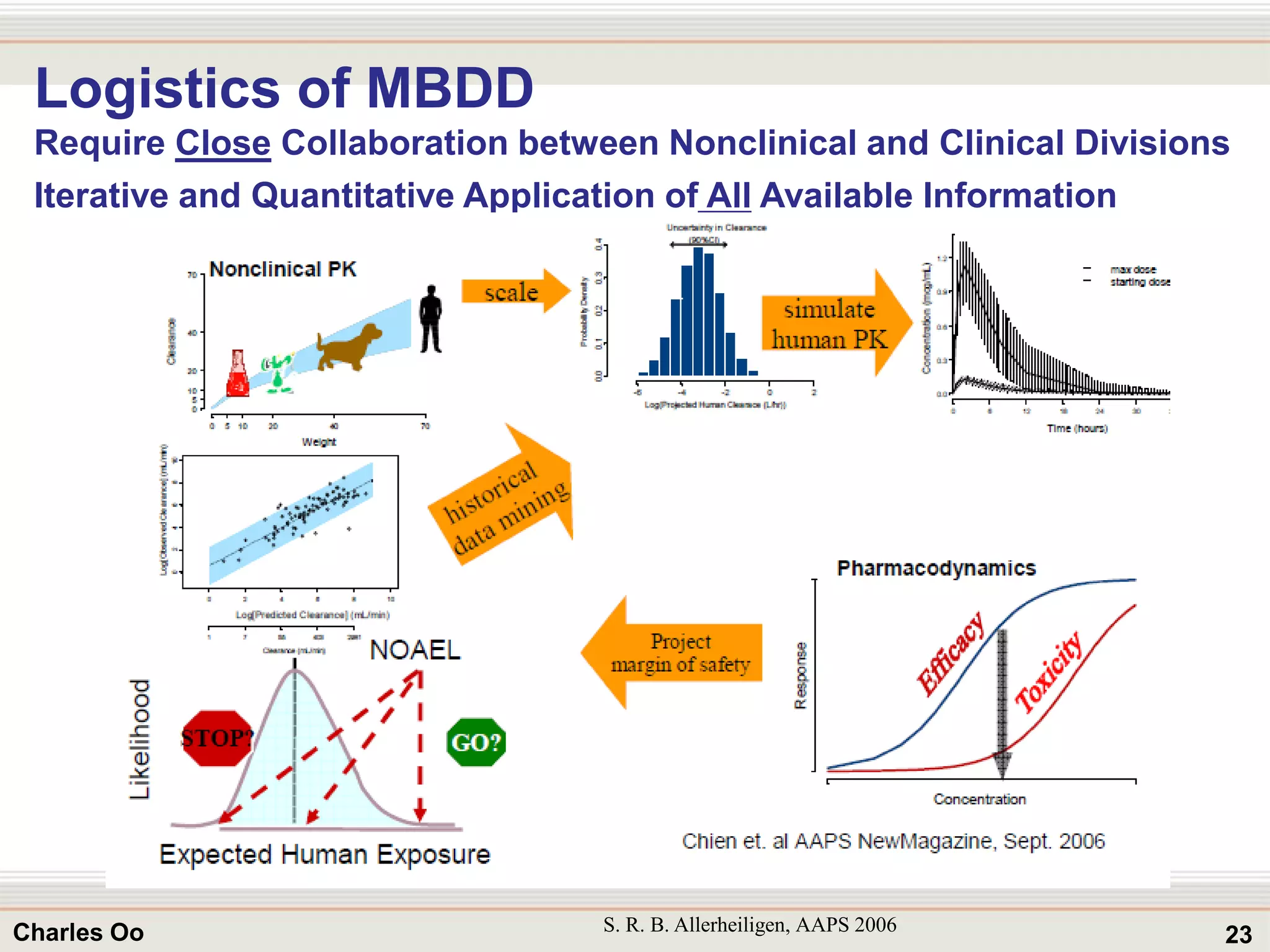
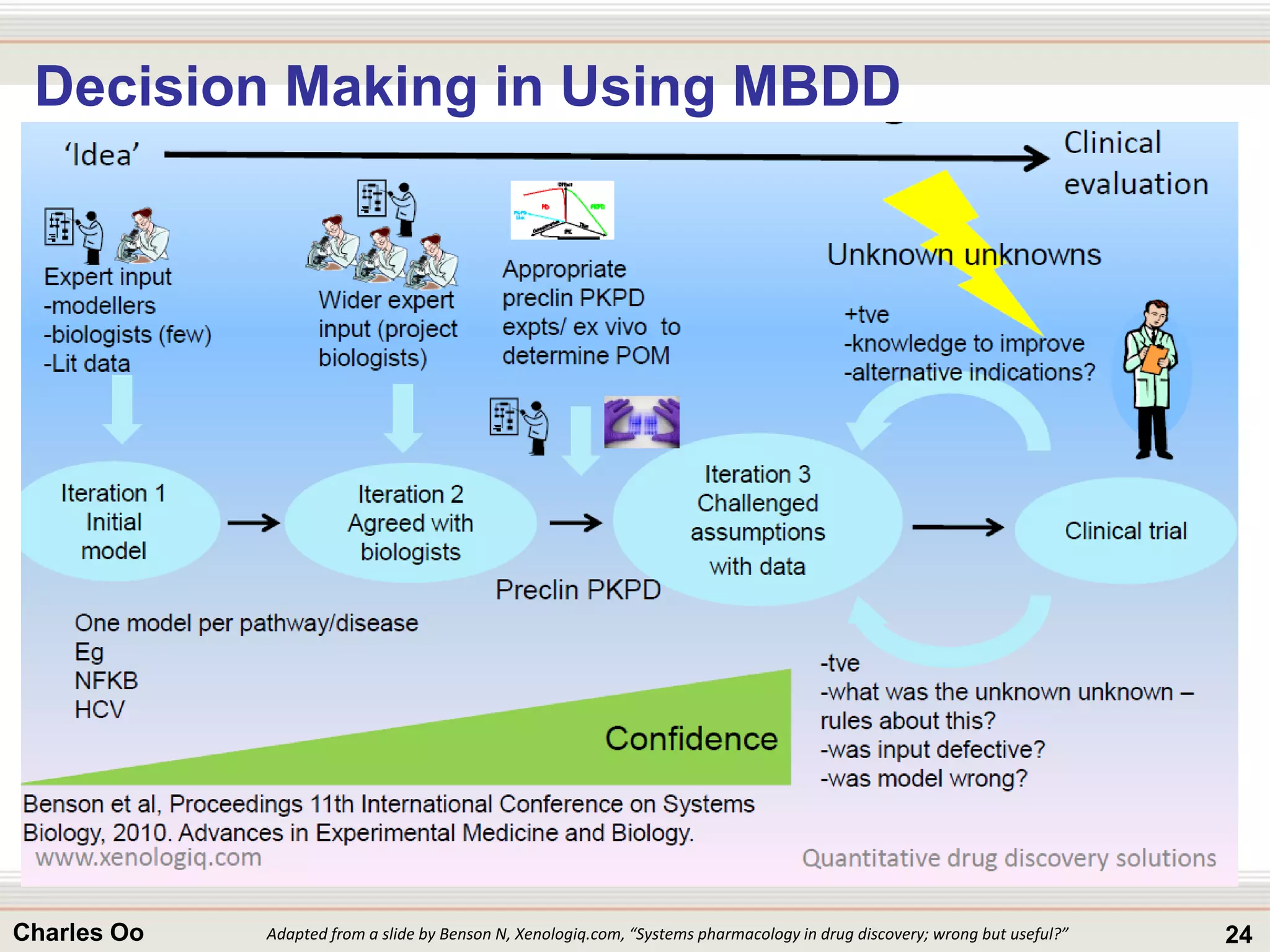
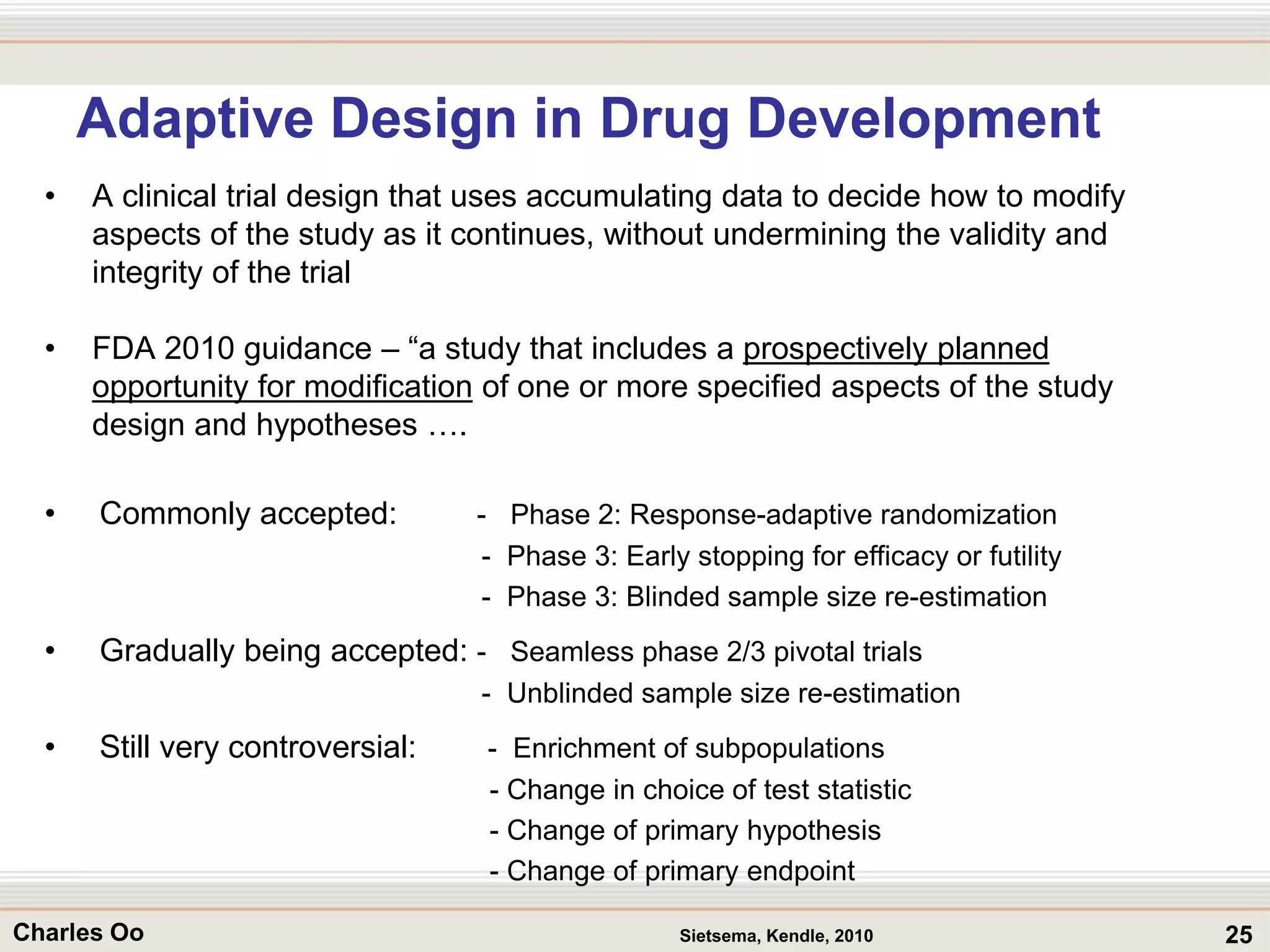
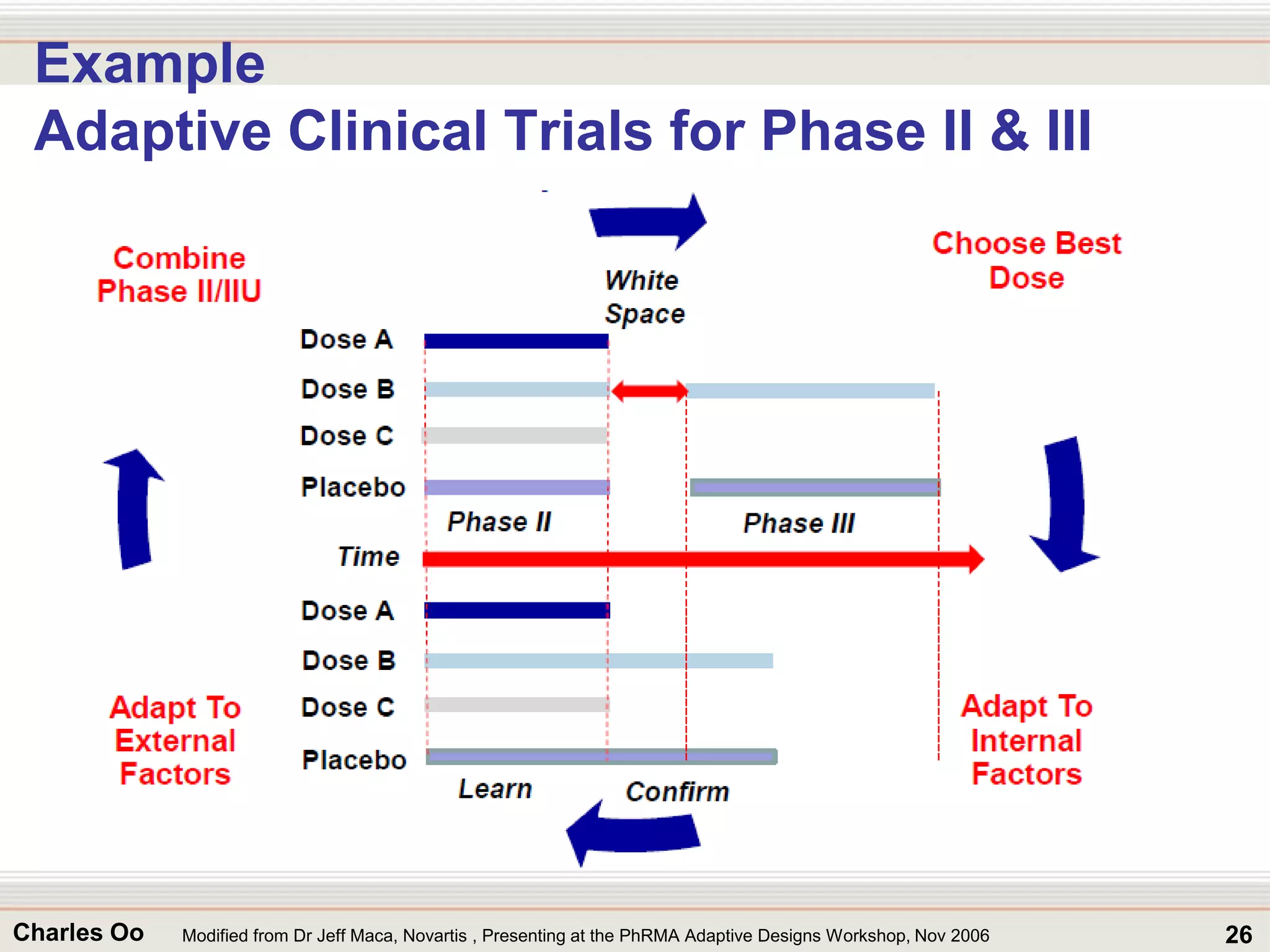
This document outlines strategies for overcoming challenges in drug development. It discusses the current long and expensive drug development process, as well as growing regulatory hurdles. It argues that innovation is needed, including open innovation models, a shift to personalized medicine, balancing drug toxicity and safety, leveraging technological advances like biomarkers, and using adaptive clinical trial designs. The key message is that new approaches are required to reduce costs, cycle times, and failure rates in drug development.