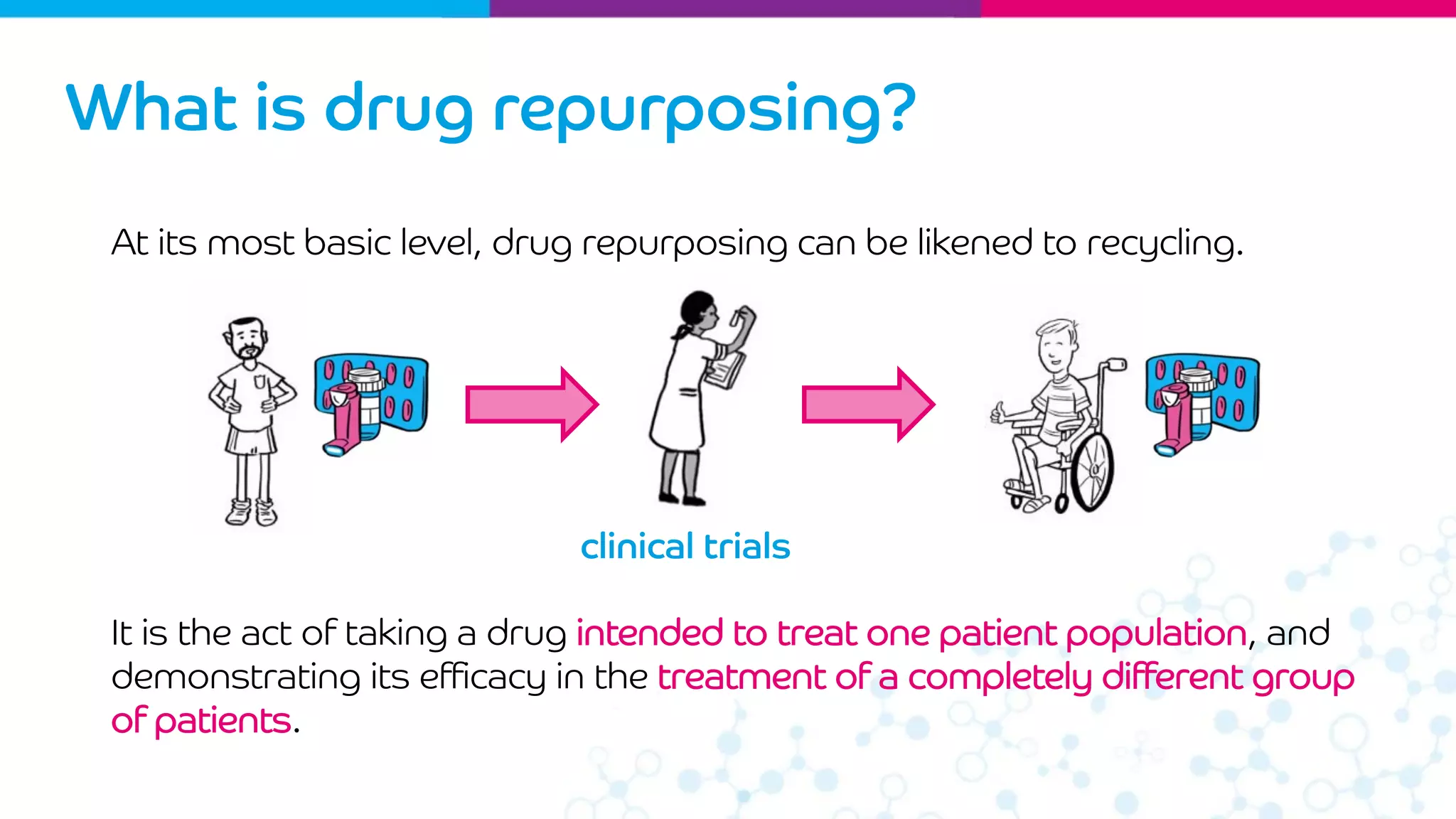
Drug repurposing involves finding new uses for existing drugs to treat rare diseases. It has advantages over developing new drugs including being faster, cheaper, and leveraging existing safety and use data. Opportunities for repurposing can be identified through screening compound libraries, literature mining, and 'omics approaches. A example is using the epilepsy drug sodium valproate identified from screening as a potential treatment for Wolfram syndrome, which is now in clinical trials.