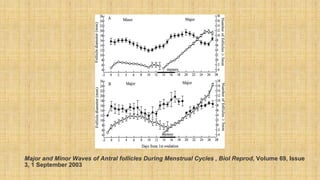
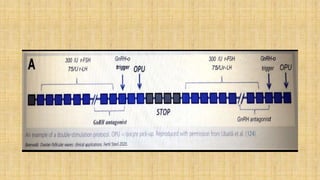
The document discusses ovarian physiology, specifically the dynamics of follicular waves during the menstrual cycle, which challenge traditional models of ovarian function and have implications for assisted reproduction. It details the concepts of random-start ovarian stimulation and double ovarian stimulation (duostim) protocols, highlighting their advantages for women with cancer or poor ovarian response. Clinical outcomes suggest that these protocols may enhance the efficiency and effectiveness of fertility treatments while having similar rates of pregnancy and live births compared to conventional methods.