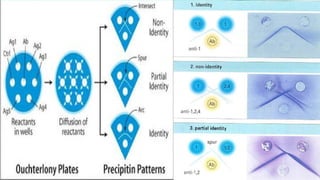
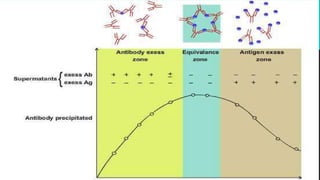
This document discusses two immunological techniques: Ouchterlony double diffusion and immunoblotting/Western blotting. Ouchterlony double diffusion involves allowing antigens and antibodies to diffuse towards each other in a gel, which can form visible precipitation lines indicating reactions. Immunoblotting involves separating proteins via gel electrophoresis, transferring them to a membrane, and using antibodies and enzyme conjugates to detect specific proteins on the membrane through colorimetric, chemiluminescent, radioactive, or fluorescent methods. Both techniques are used to detect and analyze antigens and antibodies.