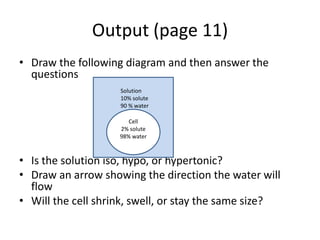
The document defines osmosis as the diffusion of water across a membrane from an area of lower solute concentration to higher solute concentration. It explains that solutions can be isotonic when solute concentrations inside and outside the cell are equal, hypotonic when outside is lower causing water to flow into the cell, or hypertonic when outside is higher causing water to flow out of the cell. Examples are given of pure water, ocean water, and a diagram is provided to determine if a solution is iso, hypo, or hypertonic based on solute concentrations and direction of water flow.


![• Note: [ ] = Concentration](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-4-320.jpg)
![• Note: [ ] = Concentration
• [Solute] + [Water] = 100%](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-5-320.jpg)
![• Note: [ ] = Concentration
• [Solute] + [Water] = 100%
• Example:
– Pure water: 0% solute, 100% water
– Ocean water: 3% solute, 97% water](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-6-320.jpg)
![Types of solutions
• Isotonic: [Solutes] in solution = [Solutes] in cell](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-7-320.jpg)
![Types of solutions
• Isotonic: [Solutes] in solution = [Solutes] in cell
• Hypotonic: [Solutes] in solution < [Solutes] in cell
Water flows IN to cell](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-8-320.jpg)
![Types of solutions
• Isotonic: [Solutes] in solution = [Solutes] in cell
• Hypotonic: [Solutes] in solution < [Solutes] in cell
Water flows IN to cell
• Hypertonic: [Solutes] in solution > [Solutes] in cell
Water flows OUT of cell](https://image.slidesharecdn.com/osmosisnotes-130109162406-phpapp01/85/Osmosis-notes-9-320.jpg)