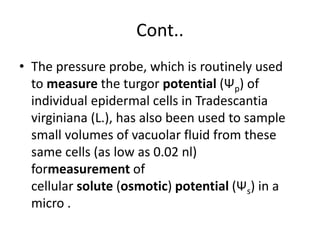
The document discusses osmotic potential, which is the potential of water molecules to move from a hypotonic solution to a hypertonic solution across a semi-permeable membrane due to differences in solute concentration. It describes how osmotic potential is always negative due to the presence of solutes, and how it is measured using a vapor pressure osmometer (VPO) which can measure samples as small as 2 microliters. The VPO provides accurate measurements of osmotic potential but has limitations for direct measurement of multiple tissue samples due to lengthy equilibration times.