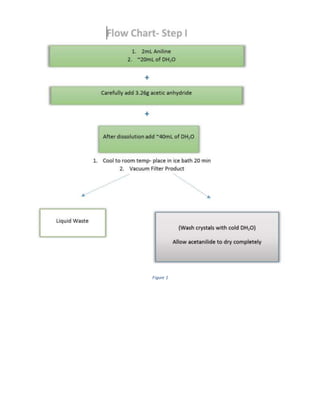
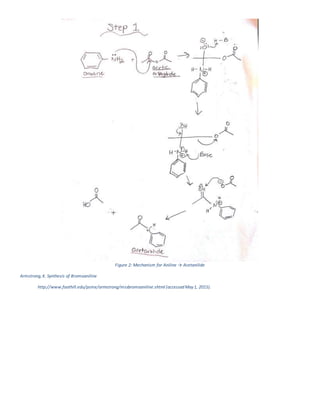
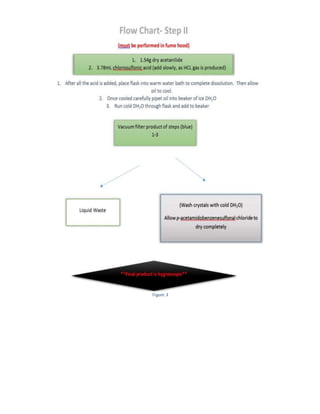
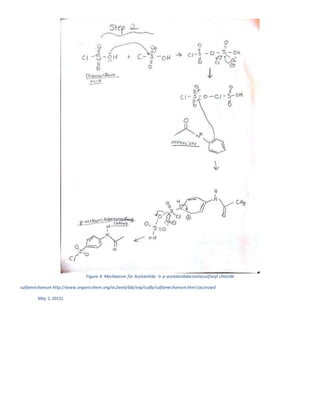
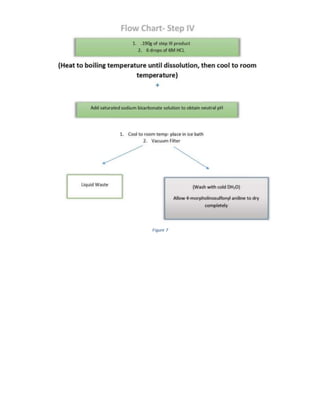
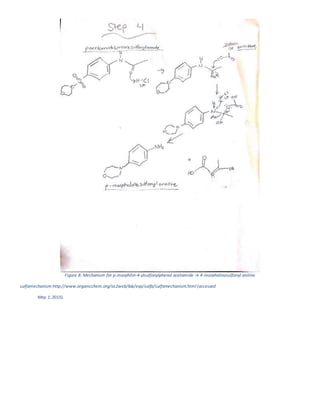
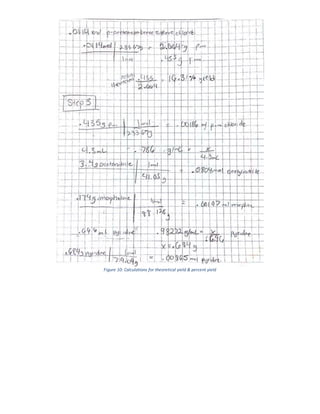
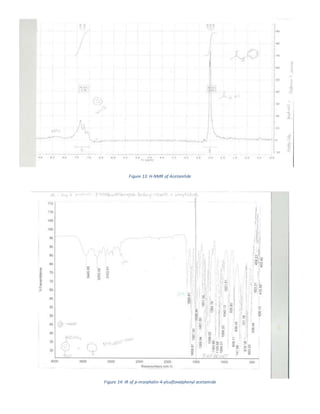
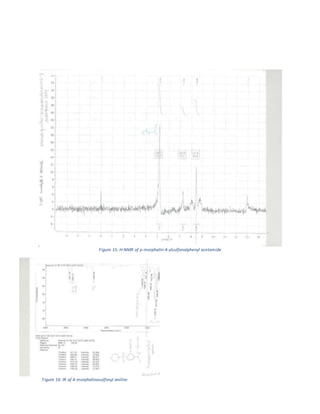
The document summarizes a 4-step synthesis of 4-morpholinosulfonyl aniline. In step 1, acetanilide was synthesized from aniline with a 67.9% yield. Step 2 involved chlorosulfonation of acetanilide to form p-acetamidobenzenesulfonyl chloride with a 16.3% yield. Step 3 yielded p-morpholin-4-ylsulfonylphenolacetamide at 60% by reacting the chloride with morpholine. Finally, step 4 hydrolyzed this product to yield the target 4-morpholinosulfonyl aniline at 32.4% yield. Spectroscopy and melting point analysis supported