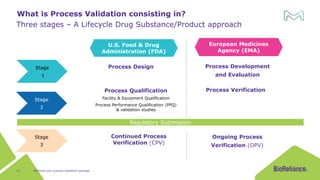
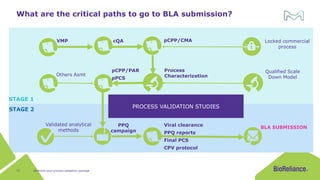
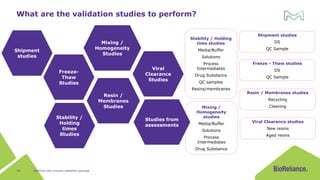
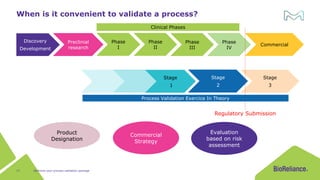
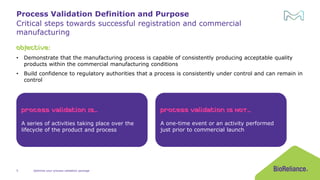
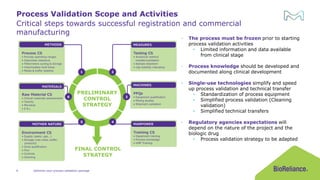
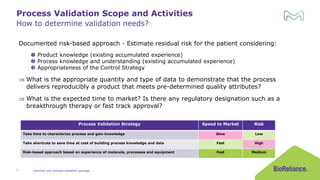
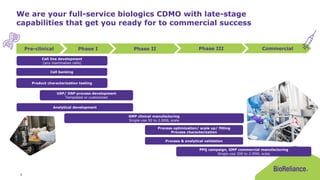
The document discusses process validation in the life science business of Merck KGaA, operating as MilliporeSigma in the U.S. and Canada, emphasizing its critical role in ensuring consistent quality in biotechnological product manufacturing. It highlights key considerations, critical success factors, the lifecycle approach to validation, and the importance of adapting strategies based on product and regulatory requirements. The presentation outlines the steps involved in successful registration and commercial manufacturing, including a risk-based approach to validation and emphasizing the use of single-use technologies.







![Optimize your process validation package
13
What is Process Validation?
A regulatory requirement
FDA guideline [1]
Process Validation is “the collection and evaluation of data, from the
process design stage through commercial production, which establishes
scientific evidence that a process is capable of CONSISTENTLY delivering
quality product”.
EMA guideline [2, 3]
“Process validation is the documented evidence that the process, operated
within established parameters, can perform EFFECTIVELY and REPRODUCIBLY
to produce an active substance or intermediate, meeting its predetermined
specifications and quality attributes”.
[1] « Guidance for Industry : Process validation : general principles and practices », FDA, 2015
[2] « Guideline on process validation for the manufacture of biotechnology-derived active substances and data to be provided in the regulatory submission », EMA, 2016
[3] « ICH Q7A Godd manufacturing practice guide for active pharmaceutical ingredients », ICH, 2010](https://image.slidesharecdn.com/processvalidationwebinar-slideshare-210624141427/85/Optimize-Your-Process-Validation-Package-13-320.jpg)