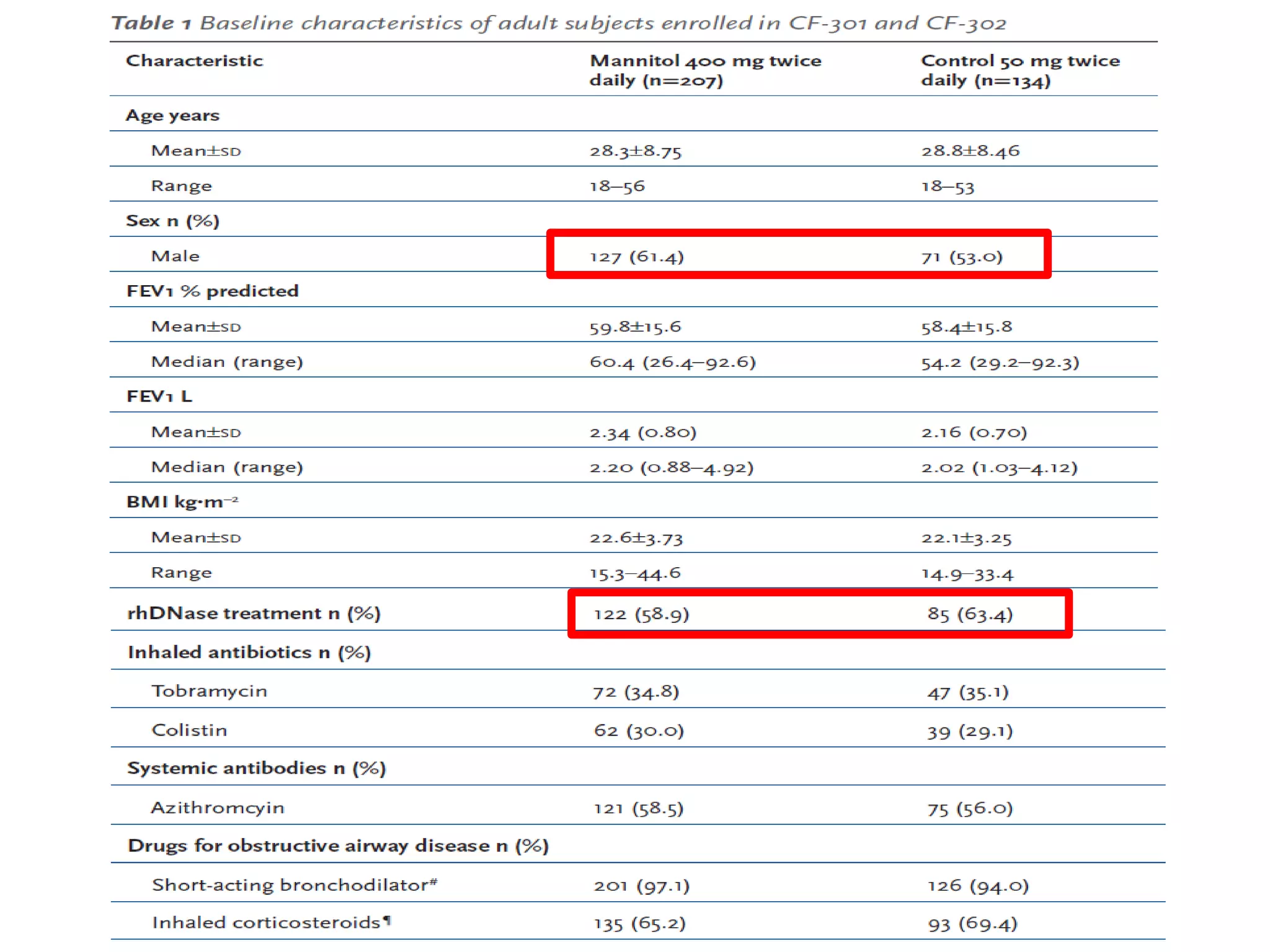
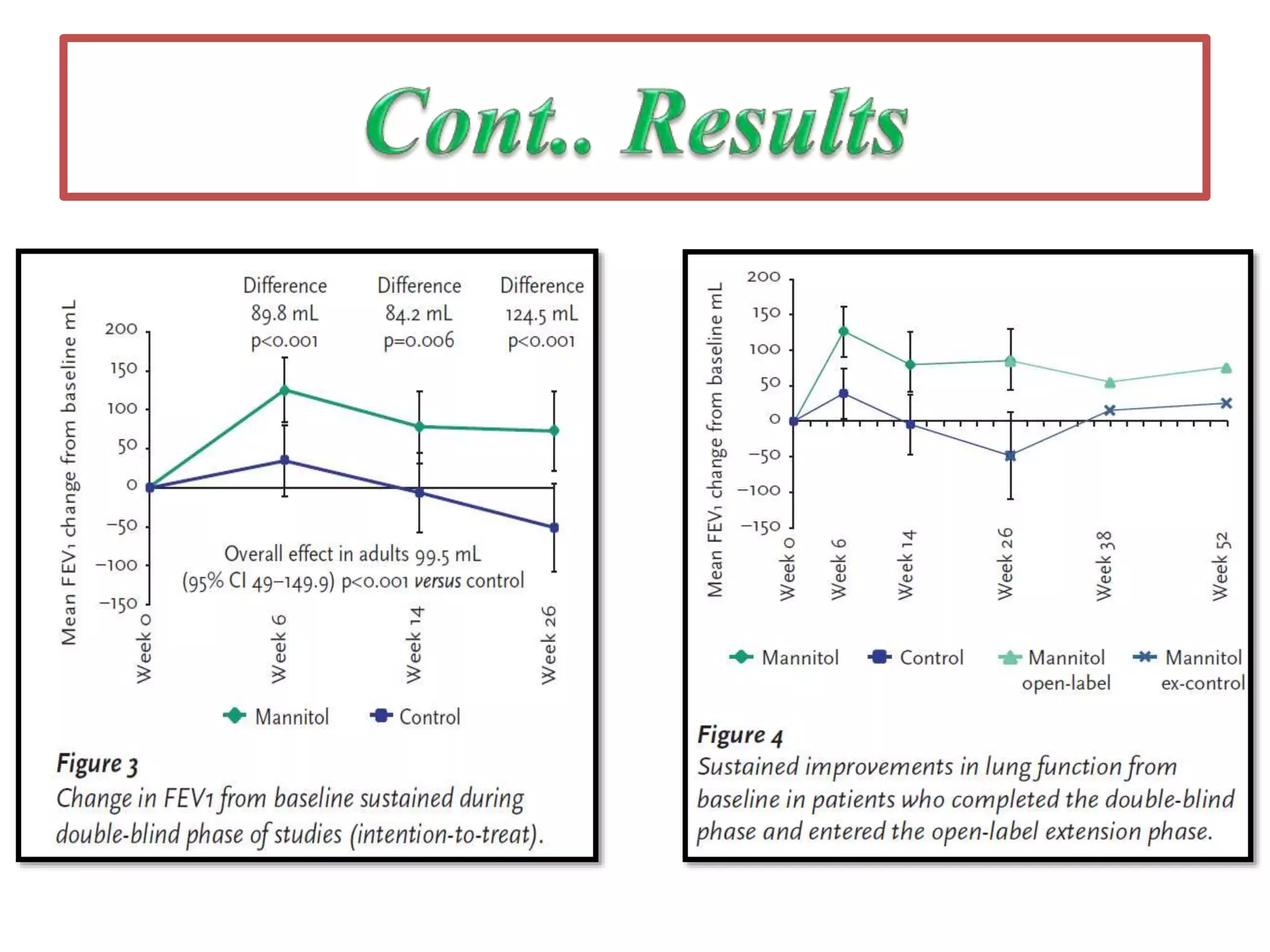
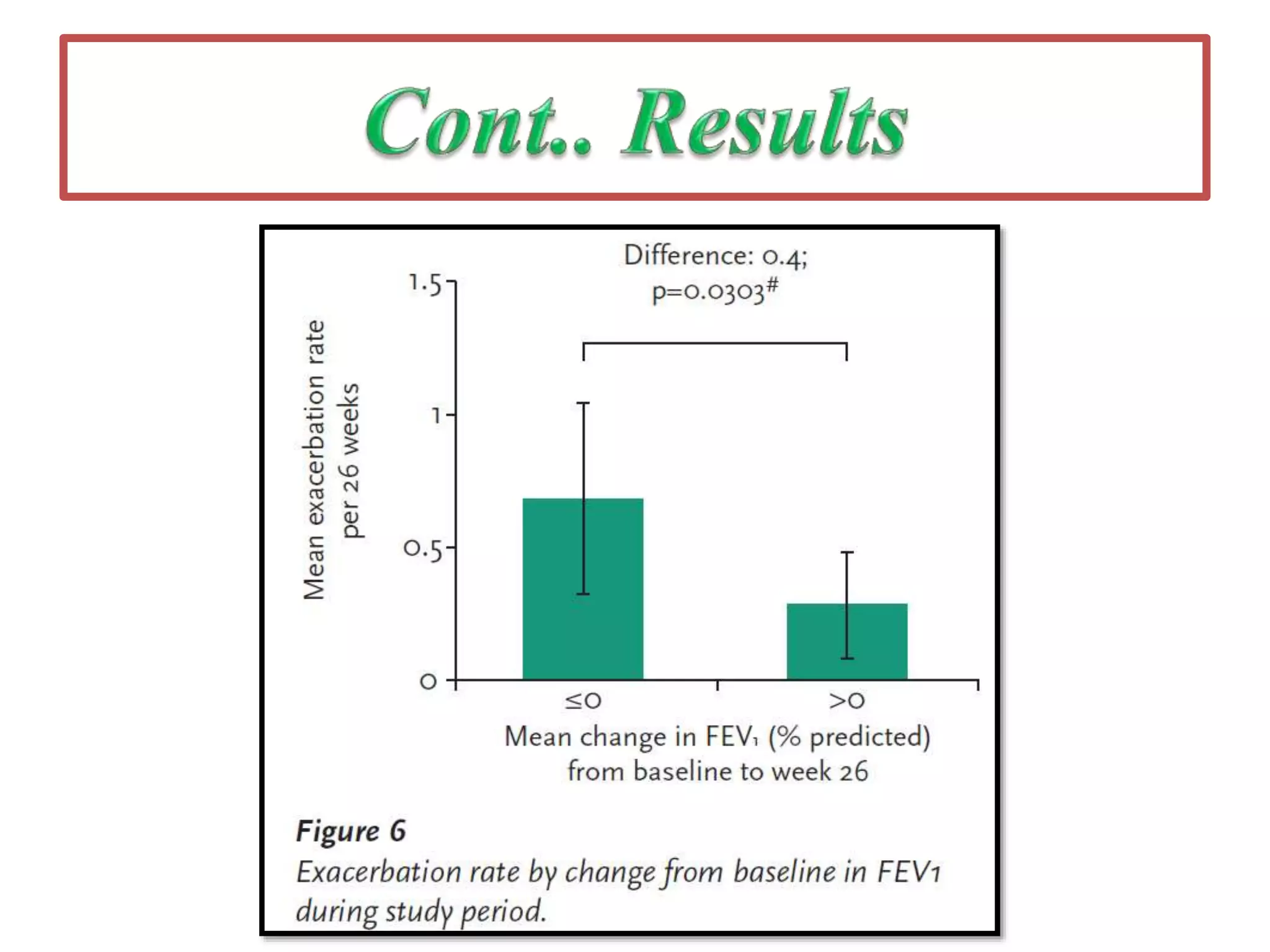
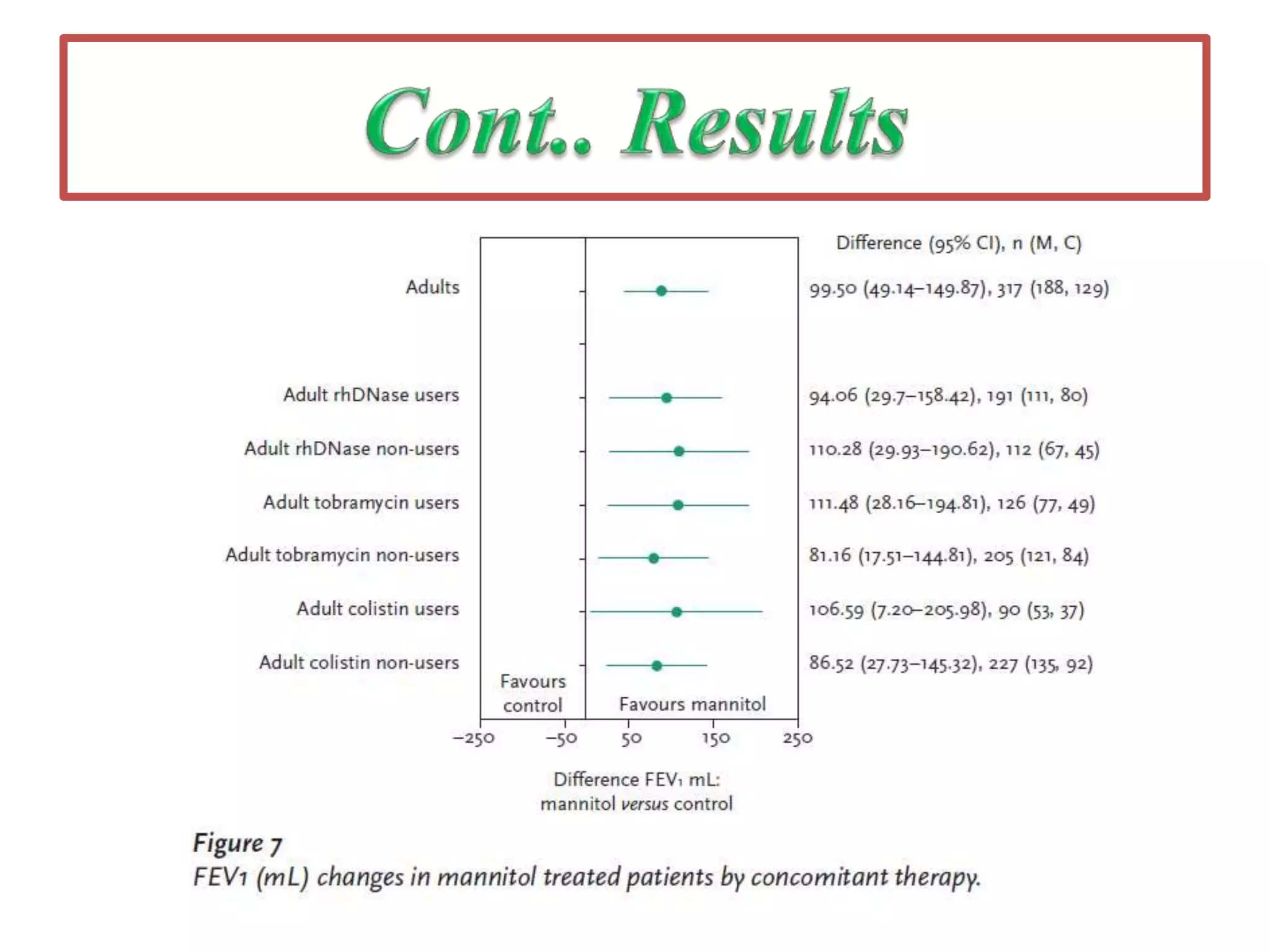
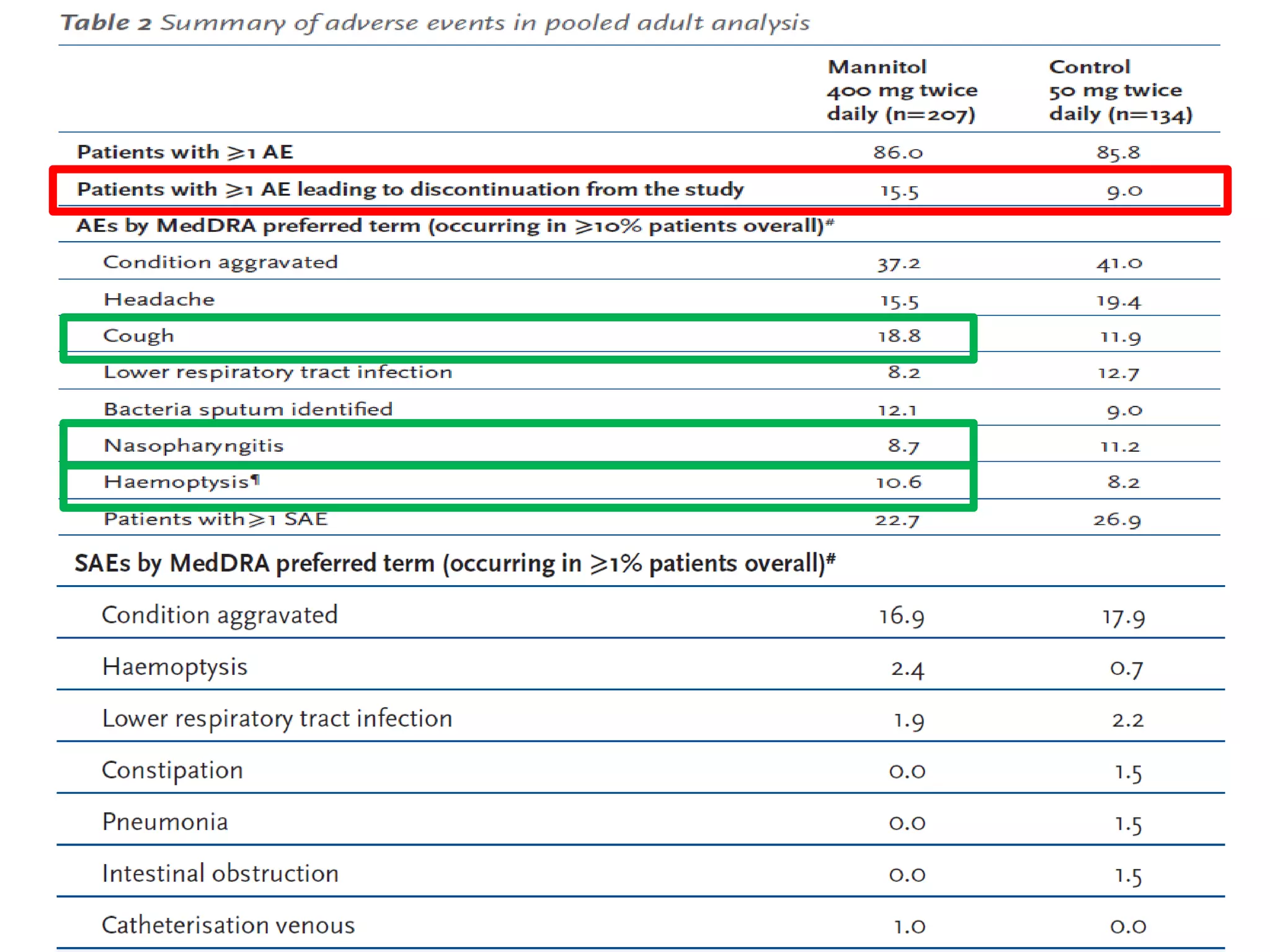
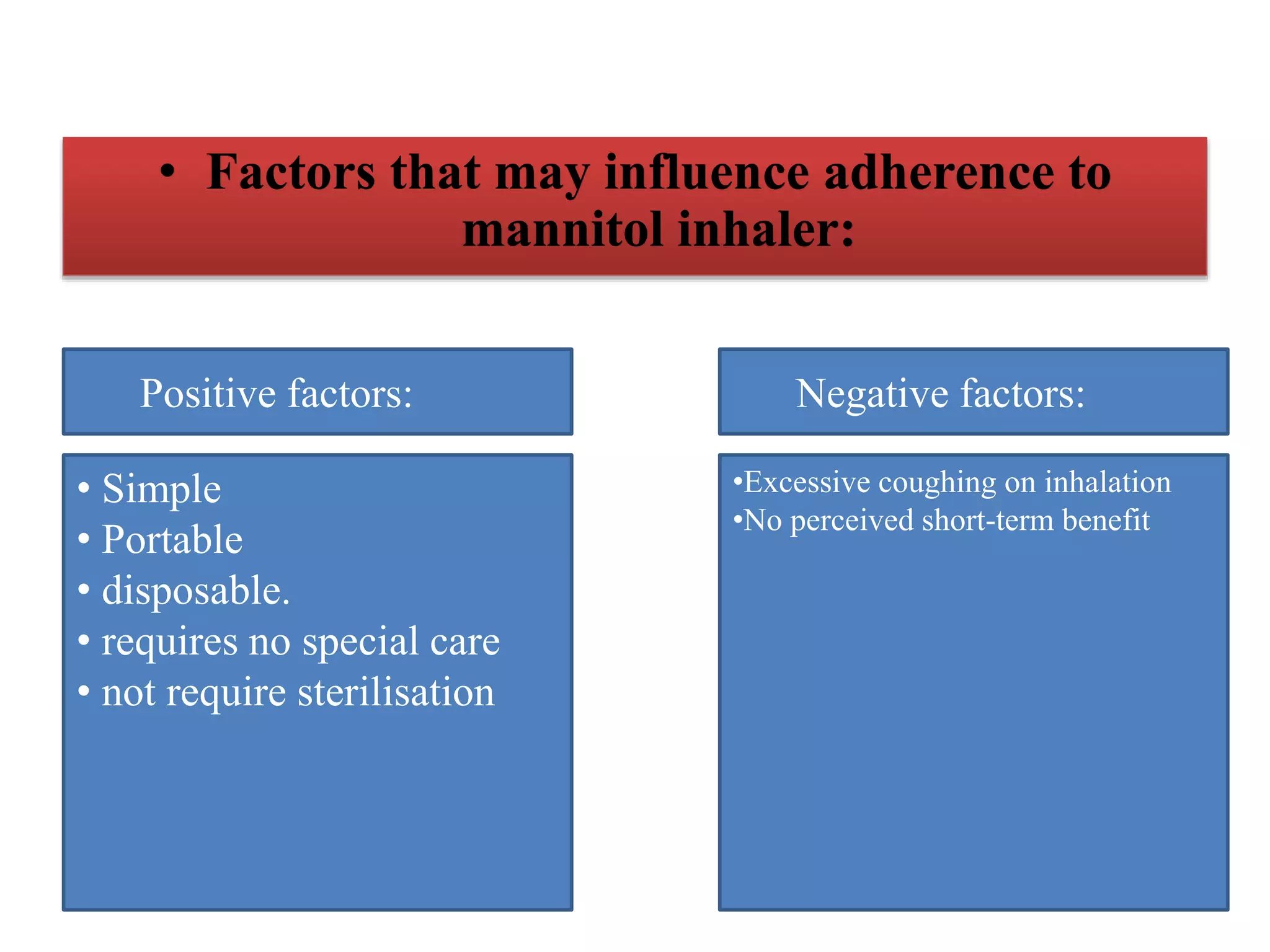
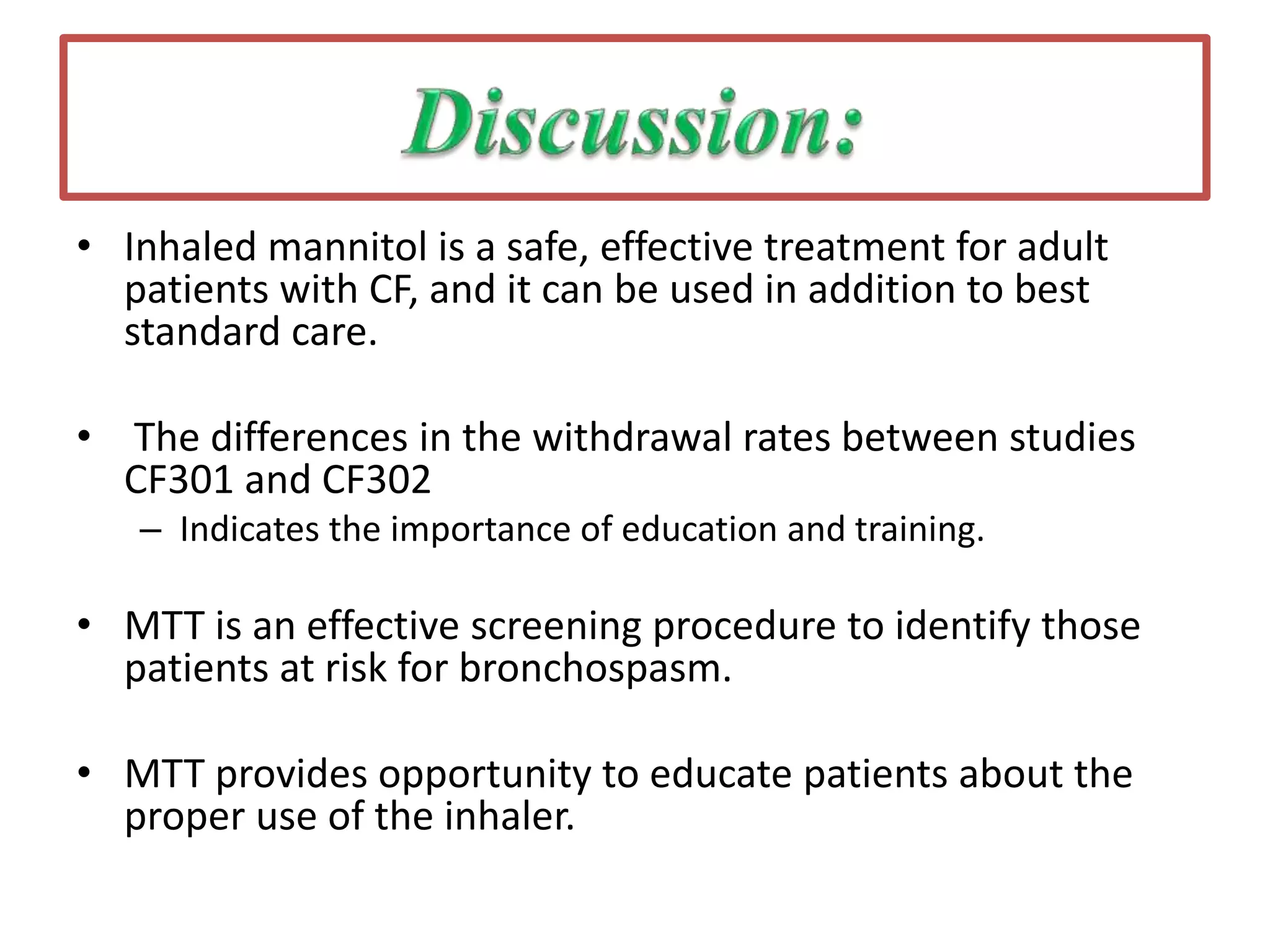
Inhaled mannitol is a new treatment approved for adults with cystic fibrosis to help clear mucus from the lungs. Two phase 3 clinical trials showed inhaled mannitol was well tolerated and led to clinically meaningful benefits over a 6 month period. Mannitol works by hydrating mucus to make it easier to cough up. While mannitol was generally well tolerated, withdrawal rates were higher in the treatment group compared to controls, indicating the importance of education and training on proper inhaler use. A mannitol tolerance test can help identify patients at risk for bronchospasm and provides an opportunity for education prior to use.