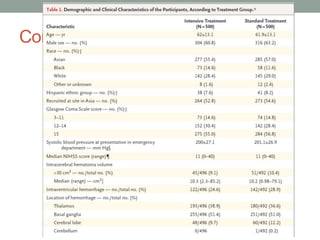
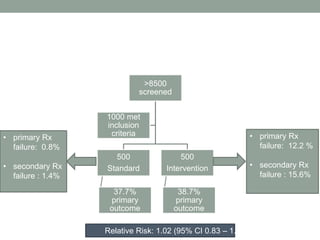
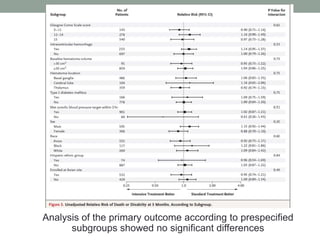
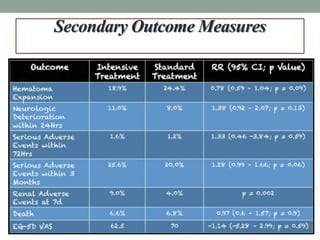
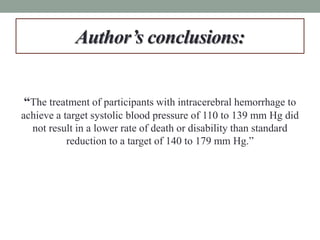
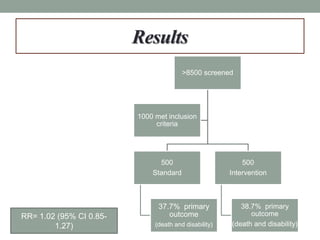
This study aimed to determine if rapidly lowering systolic blood pressure to 110-139 mmHg improves outcomes for patients with intracerebral hemorrhage, compared to standard treatment lowering it to 140-179 mmHg. Over 8500 patients were screened and 1000 were randomized to aggressive or standard treatment groups. There was no significant difference in the primary outcome of death or disability at 90 days between groups. The study suggests that intensive blood pressure reduction does not improve outcomes for intracerebral hemorrhage patients.




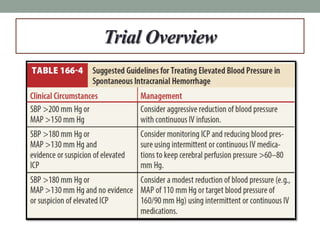
![Goals
• Primary:
• Death or disability [Modified Rankin Scale (mRS) 4-6] at 90 days
• Secondary:
• European Quality of Life–5 Dimensions (EQ-5D) questionnaire at 3
months
• > 33% expansion of hematoma at 24 hours](https://image.slidesharecdn.com/journalclub-161011200445/85/ATACH-II-trial-6-320.jpg)
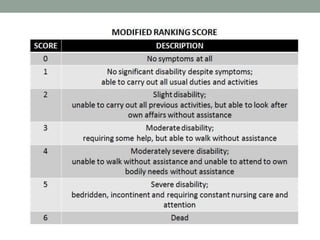






![PICO
• P:
• Patients > 18 years with intracerebral hemorrhage (< 60 cm3) and a GCS ≥5 who
presented within 4.5 hours of symptom onset and at least one blood pressure reading
with an SBP ≥180 mm Hg
• I:
• Aggressively lowering blood pressure to an SBP = 110-139 mmHg for 24 hours using
nicardipine (1st line) and labetalol, diltiazem or urapidil (2nd line)
• C:
• Standard treatment guided at a SBP = 140 – 179 mm Hg for 24 hours using nicardipine
(1st line) and labetalol, diltiazem or urapidil (2nd line)
• O:
• Primary: Death or disability [Modified Rankin Scale (mRS) 4-6] at 90 days
• Secondary: EQ-5D utility index score at 3 months, > 33% expansion of hematoma at
24 hours](https://image.slidesharecdn.com/journalclub-161011200445/85/ATACH-II-trial-18-320.jpg)