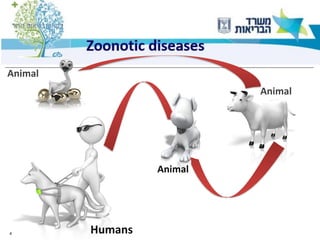
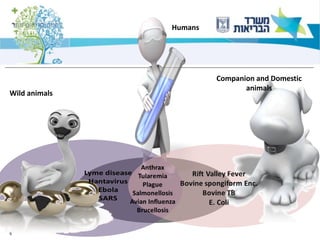
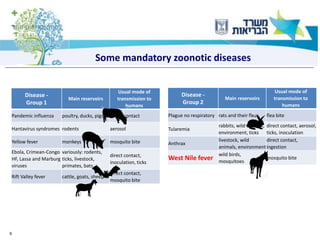
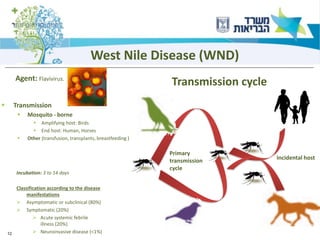
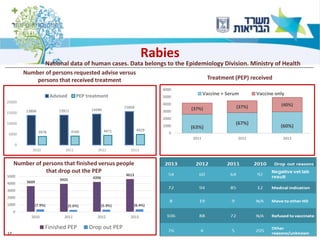
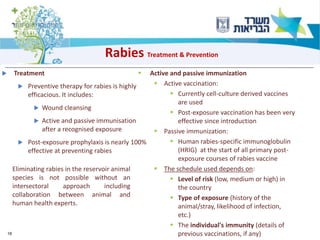
1) The document discusses several zoonotic diseases including West Nile virus, rabies, and brucellosis. It describes the pathogens, transmission cycles between animals and humans, clinical manifestations in humans, and national surveillance efforts.
2) For diseases like rabies and brucellosis, the national surveillance involves mandatory reporting of human cases, monitoring of infected animals, and collaboration between human and veterinary agencies.
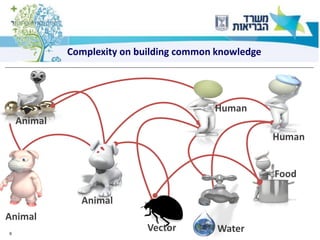
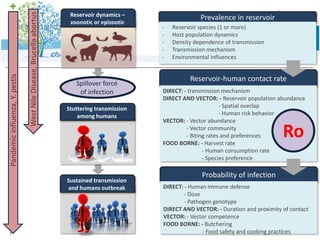
3) One health approaches discussed include integrating epidemiological data between human and veterinary fields to more rapidly detect and respond to zoonotic outbreaks.