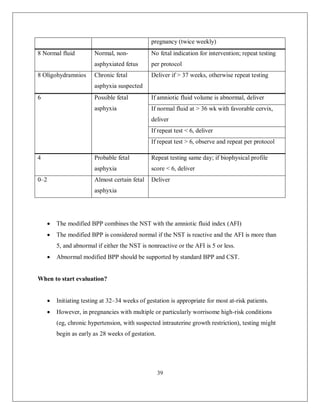
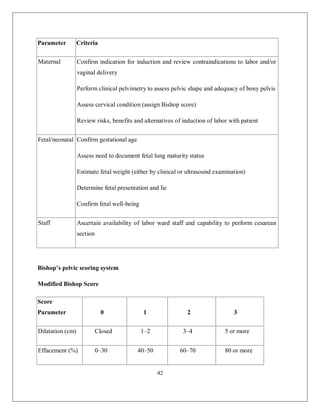
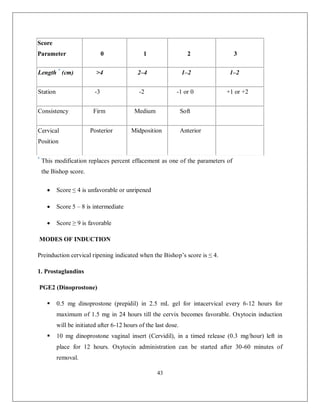
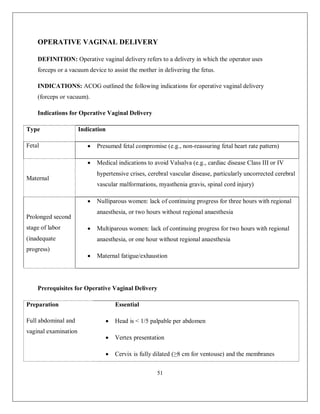
This document discusses non-reassuring fetal status (NRFS), including signs that signify fetal compromise such as repetitive decelerations or loss of beat-to-beat variability. It recommends intrauterine resuscitation initially through changing maternal positioning or treating hypotension, and delivery if the fetal heart rate does not improve. For umbilical cord prolapse (UCP), prompt delivery is necessary when the fetus is alive, through maneuvers to reduce pressure on the cord like funic decompression or cesarean delivery depending on cervical dilation and presentation. Obstructed labor is also covered, with causes like cephalo-pelvic disproportion and management including emptying the bladder, antibiotics, and relieving obstruction through procedures like
















































![53
**Minimum and maximum estimated fetal weight: Instrumental delivery of the macrosomic infant (birth weight >4000 g) may be associated with an increased risk of injury. Vacuum devices should not be used to assist delivery prior to 34 weeks of gestation (mean birth weight 2500 g) because of increased risks of fetal intraventricular hemorrhage in premature infants.
***The risks of the procedure should be explained to the woman & the informed consent discussion (with specific risks, benefits, and alternatives delineated) should be documented.
NB: The prerequisites for application of forceps or vacuum extractor are identical.
Mnemonic checklist:
Think “FORCEPS” before operative vaginal delivery:
F: The fetus is in a favorable head position, and an assessment of fetal weight and status done.
O: The patient has a completely dilated cervical OS, and the operating room is ready if needed.
R: Membranes are ruptured, and the patient qualifies for operative vaginal delivery under the rule of threes, defined as: "In an OA [occiput anterior] presentation, if the sum of the number of fifths of the fetal head palpated above the pelvic inlet abdominally and the degree of molding of the fetal head palpated vaginally equals or exceeds three, then attempted operative vaginal delivery is likely to be unsuccessful and should be avoided."
C: Contractions are present, and the patient has given consent for operative vaginal delivery.
E: The fetal head is engaged, the maternal bladder is empty, and the mother has an epidural or other anesthesia on board.
P: The maternal pelvis is adequate for operative vaginal delivery, team is prepared for cesarean delivery, and a pediatrician is available.
S: This stands for stirrups, a reminder to check that the patient is in the lithotomy position with her buttocks over the end of the bed.
Contraindications:](https://image.slidesharecdn.com/obs-141215030610-conversion-gate02/85/Obs-mx-guideline-jush-body-53-320.jpg)
























![78
The 100-g 3-hour oral glucose tolerance test performed after an overnight fast remains the standard.
Oral GTT [*] National Diabetic plasma Data Group (NDDG)
Fasting 105
1-hour 190
2-hour 165
3-hour 145
* Diagnosis of gestational diabetes is made when any two or more values are met or exceeded. If only one value is met, the test be repeated in a month’s time.
Treatment of GDM
The mainstay of treatment is:
Nutritional counseling
Exercise?
Dietary intervention and
Surveillance of blood glucose levels (weekly FBS/ postprandial)
Initiate insulin if:
Fasting glucose level is above 105 mg/dL or 2 hours postprandial is >120 mg/dL after 2 weeks of non-drug treatment.
Glucose monitoring
Frequent self–blood glucose monitoring is fundamental to achieve the therapeutic objective of physiologic glucose control.
Glucose determinations are made in the fasting state and before lunch, dinner, and bedtime.](https://image.slidesharecdn.com/obs-141215030610-conversion-gate02/85/Obs-mx-guideline-jush-body-78-320.jpg)








































































