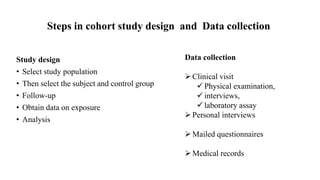
This document discusses different types of observational studies, including cohort studies, case-control studies, and cross-sectional studies. Cohort studies follow groups of individuals over time to determine outcomes. Case-control studies compare individuals with a disease (cases) to those without (controls) to identify potential risk factors. Cross-sectional studies collect data on exposure and outcome at the same time to determine prevalence. While each study type has advantages and disadvantages, together they help identify disease risk factors and generate hypotheses for further research.