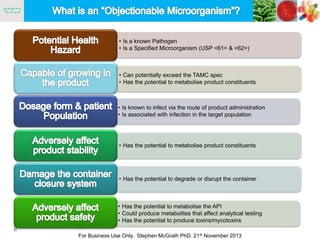
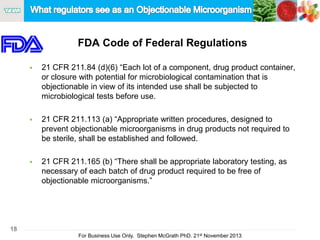
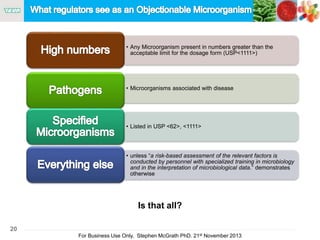
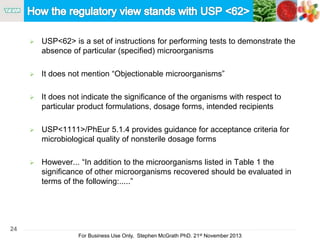
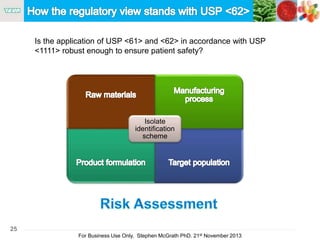
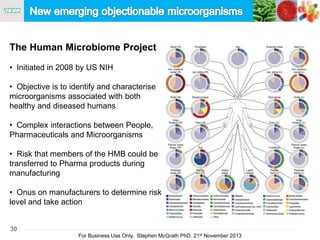
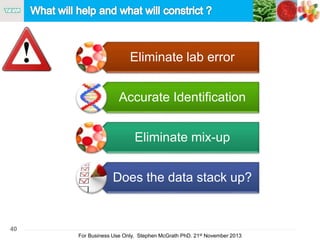
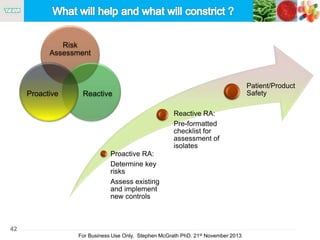
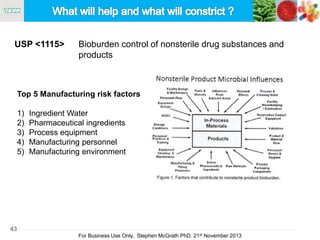
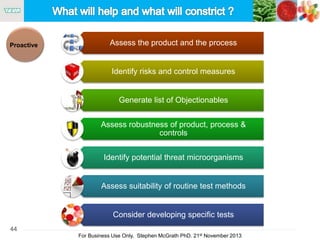
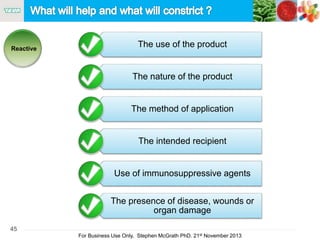
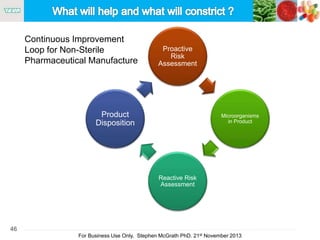
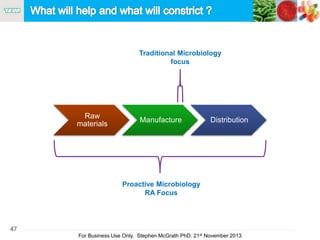
The document discusses the concept of objectionable microorganisms in non-sterile pharmaceutical products, emphasizing the manufacturer's responsibility to ensure product safety. It outlines the definition of objectionable microorganisms, relevant regulatory frameworks, and historical insights into microbial testing practices. The content highlights the importance of risk-based assessments and proactive microbiological controls to prevent contamination and ensure consumer safety.