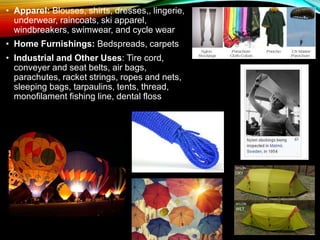
The document is a presentation from a group of students in the Department of Fashion Design and Technology at Uttara University, focusing on textile raw materials like nylon and viscose rayon. It includes details on the general information, manufacturing processes, chemical and physical properties, and uses of these materials. Acknowledgments are expressed to the faculty for guidance and support throughout the project.






![INTRODUCTION ABOUT NYLONE
• First created by William Carother, a
Lecturer from Harvard University, in
1934 at DuPont Company research lab
• DuPont scientist created Nylon 6.6
• German scientist created Nylon 6
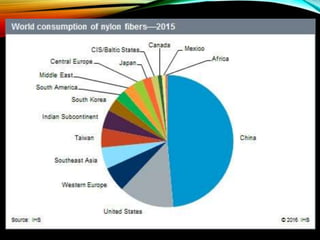
• Second most used fiber in the U.S.A
• First used in a nylon toothbrush
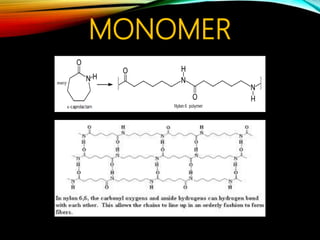
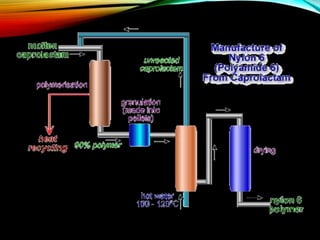
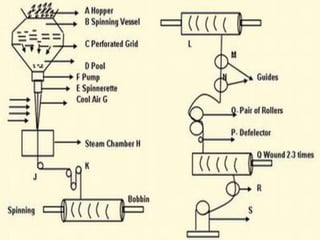
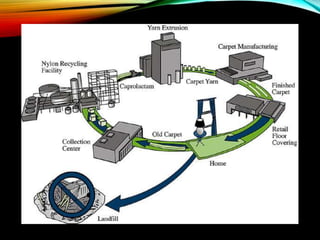
Nylon is a generic designation for a family of synthetic
polymers, based on aliphatic or semi-aromatic polyamides.
Nylon is a thermoplastic silky material[1] that can be melt-
processed into fibers, filmsor shapes.](https://image.slidesharecdn.com/nylonandviscos-181113072039/85/Nylon-and-viscos-9-320.jpg)








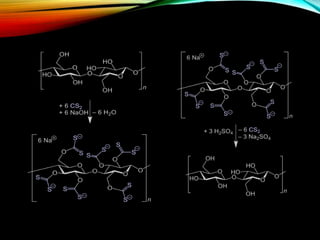
![MONOMER
• Viscose rayon is a fiber of regenerated cellulose; it is
structurally similar to cotton but may be produced from a
variety of plants such as soy, bamboo, and sugar cane.
Cellulose is a linear polymer of β-D-glucose units with the
empirical formula (C6H10O5)n.[2] To prepare viscose, dissolving
pulp is treated with aqueous sodium hydroxide (typically 16-
19% w/w) to form "alkali cellulose," which has the
approximate formula [C6H9O4-ONa]n. The alkali cellulose is
then treated with carbon disulfide to form sodium
cellulose xanthate.[3]
• [C6H9O4-ONa]n + nCS2 → [C6H9O4-OCS2Na]n](https://image.slidesharecdn.com/nylonandviscos-181113072039/85/Nylon-and-viscos-26-320.jpg)
![• Rayon fiber is produced from the ripened solutions by
treatment with a mineral acid, such as sulfuric acid. In this
step, the xanthate groups are hydrolyzed to regenerate
cellulose and release dithiocarbonic acid that later
decomposes to carbon disulfide and water:[5]
• [C6H9O4-OCS2Na]2n + nH2SO4 → [C6H9O4-OH]2n +2nCS2 +
nNa2SO4
• H2COS2 → H2O + CS2](https://image.slidesharecdn.com/nylonandviscos-181113072039/85/Nylon-and-viscos-27-320.jpg)





![• Rayon or artificial silk is a manufactured regenerated cellulose
fiber.
• It is made from purified cellulose, primarily from wood pulp, which
is chemically converted into a soluble compound. It is then
dissolved and forced through a spinneret to produce filaments
which are chemically solidified, resulting in synthetic fibers of
nearly pure cellulose.[1]
• Workers can be seriously harmed by the carbon disulfide used to
make most rayon.[2][3]
• Because rayon is manufactured from naturally occurring polymers,
it is considered a semi-synthetic fiber.[4] Specific types of rayon
include viscose, modal and lyocell, each of which differs in
manufacturing process and properties of the finished product.](https://image.slidesharecdn.com/nylonandviscos-181113072039/85/Nylon-and-viscos-34-320.jpg)

![• The durability and appearance retention of regular viscose
rayon are low, especially when wet; also, rayon has the
lowest elastic recovery of any fiber. However, HWM rayon
(high-wet-modulus rayon) is much stronger and exhibits
higher durability and appearance retention. Recommended
care for regular viscose rayon is dry-cleaning only. HWM
rayon can be machine washed.[10]
• Rayon industrial yarns outperform polyester and are
produced for belts in high performance tires (e.g. Cordenka,
Germany).](https://image.slidesharecdn.com/nylonandviscos-181113072039/85/Nylon-and-viscos-36-320.jpg)




