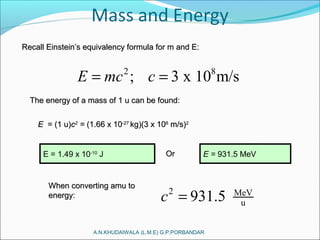
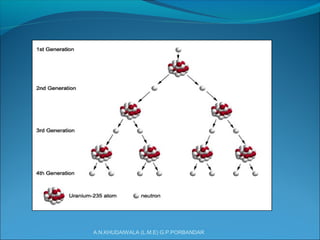
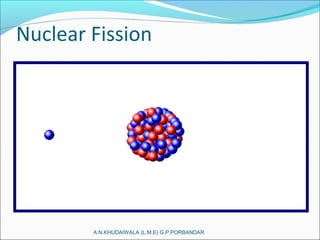
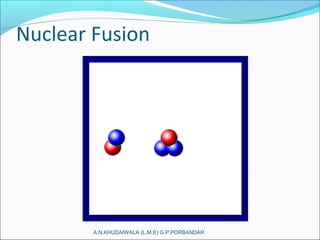
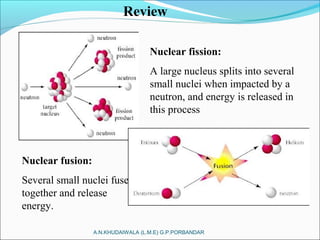
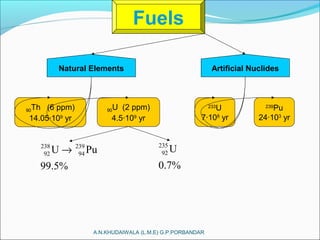
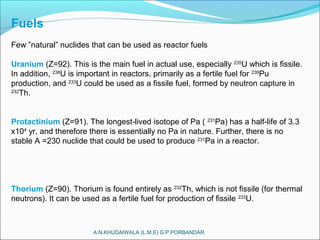
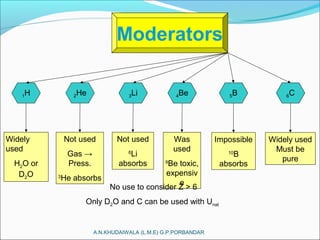
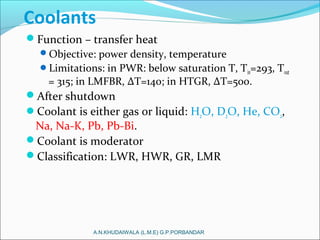
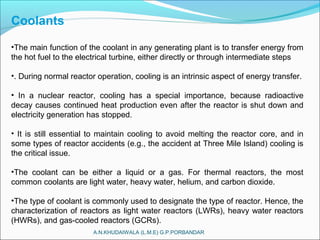
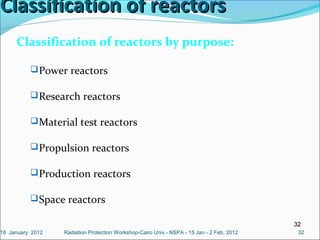
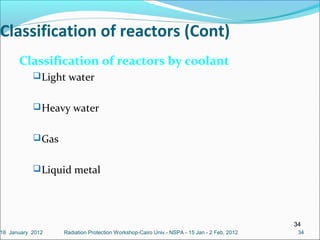
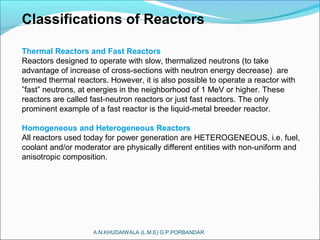
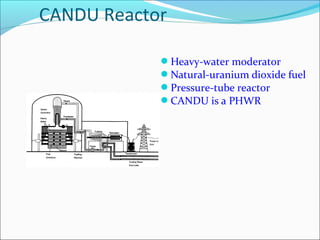
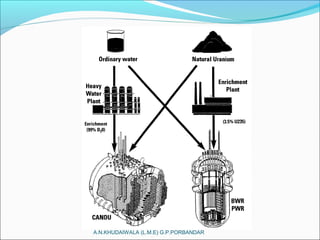
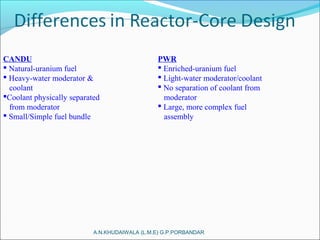
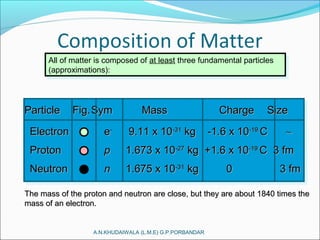
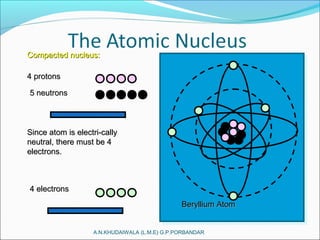
The document covers fundamental concepts of nuclear physics, including mass number, atomic number, isotopes, radioactive decay, and nuclear reactions. It details the processes of nuclear fission and fusion, outlining how these processes can be harnessed in nuclear reactors and weapons. The document also discusses the classification of nuclear reactors, essential components such as fuel and moderators, and the importance of cooling systems in reactor safety.


![A.N.KHUDAIWALA (L.M.E) G.P.PORBANDAR
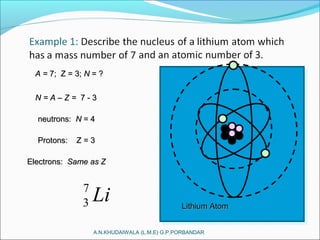
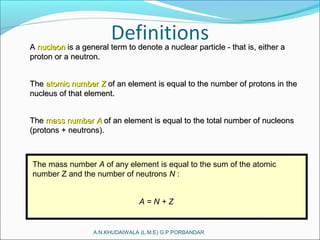
A convenient way of describing an element is by giving its mass number andA convenient way of describing an element is by giving its mass number and
its atomic number, along with the chemical symbol for that element.its atomic number, along with the chemical symbol for that element.
A convenient way of describing an element is by giving its mass number andA convenient way of describing an element is by giving its mass number and
its atomic number, along with the chemical symbol for that element.its atomic number, along with the chemical symbol for that element.
[ ]A Mass number
Z Atomic numberX Symbol=
For example, consider beryllium (Be): 9
4 Be](https://image.slidesharecdn.com/nuclearpowerplant-161230114739/85/Nuclear-power-plant-6-320.jpg)