This document provides information about alpha decay and alpha particles. It discusses:
1) Unstable nuclei attain stability through emission of alpha particles, which are made up of 2 protons and 2 neutrons.
2) Alpha decay involves the emission of an alpha particle from an unstable nucleus, leaving a lighter nucleus. Conservation laws apply.
3) The range of alpha particles is very small, usually only a few centimeters in air or solid materials, due to their high ionization which causes energy loss. Their short range makes them easily stopped.

![ALPHA DECAY
INTRODUCTON:
Nuclei with certain combination of protons and neutrons have unstable configuration.
[radioactive]
Unstable nuclei attain stability by emission of certain particles.
α- decay: The emission of α-particles by a unstable radioactive nuclei.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-2-320.jpg)
![Alpha particle:
a positively charged particle emitted by a radioactive nucleus.
made up of 2 protons and 2 neutrons.
α-particle is the nucleus of a helium atom [2He4].](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-3-320.jpg)

![Energetics of Spontaneous Alpha Decay:
The process of alpha decay is given as:
ZXA
Z-2YA-4 + 2He4
where Y is the residual nucleus of mass number A-4 are the parent and atomic number Z-2.
If the masses of the α-particle [2He4] and the residual nucleus be 𝑀𝛼 and 𝑀1 respectively, and
𝑣𝛼 and 𝑣1 their respective velocities then conservation of momentum requires that:
𝑀𝛼𝑣𝛼 = 𝑀1𝑣1------------------------------------------------------------[1]](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-5-320.jpg)
![ If Q is the α-disintegration energy, which is the total energy released in the disintegration
process,
Q =
1
2
𝑀𝛼𝑣α
2
+
1
2
𝑀1𝑣1
2
Using equation [1] and on simplification, we get:
Q = 𝐸𝛼
𝑀1+ 𝑀α
𝑀1](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-6-320.jpg)
![Masses of the nuclei in the unit of atomic masses are close to their mass numbers, we can
write 𝑀1= A-4 and 𝑀𝛼 = 4.
Alpha disintegration energy-
Q = 𝐸𝛼
𝐴
𝐴−4
----------------------------------------------------------------[2]
Q can be determined from equation [1] as 𝐸𝛼 can be determined experimentally.
Example: 210Po, the α disintegration energy is 𝐸𝛼 = 5.305 MeV which gives Q = 5.408 MeV.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-7-320.jpg)






![Experiment to measure the Range of α–particle:
W.H.Bragg [England] – first to determine the range of α–particle.
Measured the ionization produced by α–particle at different distances from the sources along
their path within the medium.
Experimental set-up:
α–particles produced by the source S are collimated by a slit within the plate P.
A and B are two parallel wire-gauzes with a very small gap between them.
The positions of these wire-gauzes can be changed without altering the distance between
them.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-14-320.jpg)


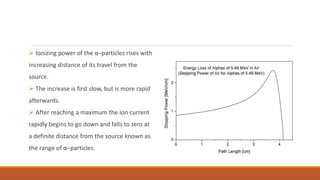

![SPECIFIC IONIZATION:
The number ∆𝑛
∆𝑥 of ion-pairs produced per unit length of its travel by an α–particle in a gas at
one atmospheric pressure.
∆𝑛 is number of ion-pairs produced in a distance ∆𝑥.
The specific ionization depends on the distance travelled by the α–particle from the source.
Example: For RaC’ α–particles [E = 7.68 MeV], the maximum value of the specific ionization is
about 6000 ion-pairs per millimetre.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-20-320.jpg)




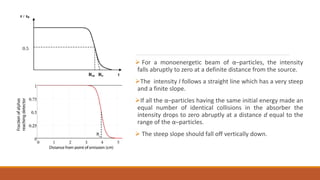

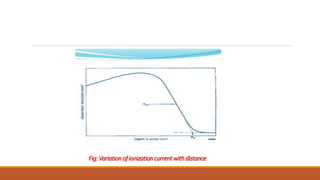
![MEAN RANGE [R]: The distance from the source at which the intensity is half the initial
intensity which is a few millimetres shorter than Rex.
The mean range has a value such that 50% of the α–particles in the incident beam have ranges
greater than R while 50% have ranges less than R.
For a given energy of the incident α–particles, the different ranges defined above have slightly
different values.
Example: 210Po α–rays [𝐸𝛼 = 5.3007 MeV], the following values were obtained:
Ionization extrapolated range 𝑅𝑖 = 3.870 cm, extrapolated range 𝑅𝑒𝑥 = 3.897 cm, Mean range:
3.842cm.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-27-320.jpg)

![The relationship for the range in terms of velocity:
R = b𝑣3
where b is another constant: b = 9.416 x 10−28
In the case of a solid, the range Rs[ in centimetre] is related to the range R in air as follows:
𝑅𝑠 =
0.312 𝑅𝐴1/2
𝜌
where 𝜌 is the density of solid of mass number A.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-29-320.jpg)
![Geiger-Nuttall law
Geiger-Nuttall [1911] discovered an empirical relationship between the ranges of the α–
particles and the disintegration constants of the naturally radioactive substances emitting them.
This is known as Geiger-Nuttall law.
The relationship is given by:
log 𝝀 = A + B log R. ----------------------------------------------------[1]
where A and B are constants.
According to this law, the α–particles emitted by the substances having larger disintegration
constants [shorter half-lives] have longer ranges and vice-versa.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-30-320.jpg)
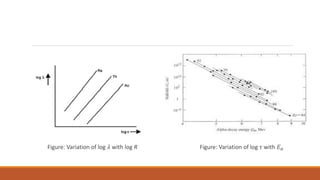
![Equation [1] shows the graph of log 𝜆 and log R is a straight line with a slope B.
For different radioactive series, different straight lines are obtained, which are parallel to one
another, so that B is same for all of them.
Using equation [1] it is possible to determine the disintegration constant and the half life 𝜏 of a
radioactive substance if the range of α–particles emitted by it is known accurately.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-31-320.jpg)


![IMPORTANT NOTE:
The ranges of the α–particles from different radioactive isotopes are of the order of few
centimetres in air.
The half-lives of the radioactive isotope range from less than a millionth of a second to more
than billion[109
] years.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-35-320.jpg)

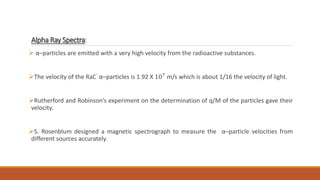

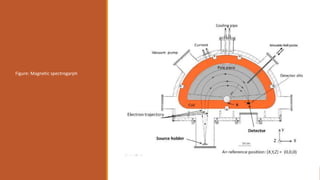

![Rosenblum used magnetic induction fields up to 3.6T [36,000] gauss.
Results:
The velocities of the α–particles emitted by the radioactive substances are of the order of
107
m/s.
For some radio-elements, only one line is obtained in the α–spectrum on the photographic
plate, which shows that they emit α–particles of a single velocity.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-41-320.jpg)
![In some cases a number of parallel lines separated from one another [discrete spectrum] are
obtained, which shows that a number of different mono-energetic groups of α–particles are
emitted by the substances.
Each group has a definite velocity.
The velocities of the different groups are different.
The kinetic energies of the α–particles emitted from naturally radioactive substances are usually
in the range of 4 to 10 MeV.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-42-320.jpg)
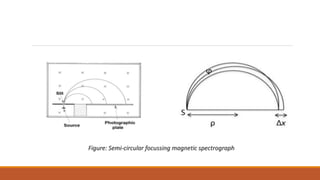

![ By measurements of 𝛾-energy and α–energy, the energy levels of the nucleus can be found.
Rosenblum showed by using semi-circular focussing magnetic spectrograph that the α–rays
from ThC [𝐵𝑖212] are not monoenergetic.
It consists of several closely spaced mono-energetic groups or α–ray lines](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-45-320.jpg)
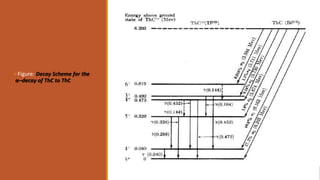
![Figure: Discrete spectra [Example]](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-46-320.jpg)
![The interpretation of complex α–particle spectra in terms of nuclear energy levels is illustrated
by the case of ThC [ Bi212].
It emits six groups of short-range α–particles with energies shown in the figure.](https://image.slidesharecdn.com/alphadecay-210422081000/85/Nuclear-physics-47-320.jpg)