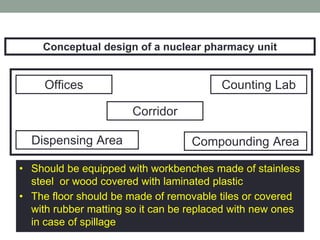
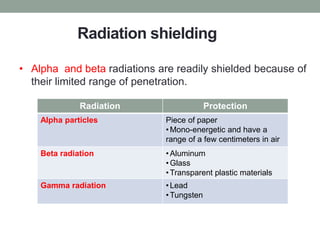
Nuclear pharmacy is a specialty area of pharmacy practice that requires expertise in radiopharmaceuticals for nuclear medicine procedures. Nuclear pharmacists work in hospitals, pharmacies, academia, and research to prepare, dispense, and quality test radiopharmaceuticals used for diagnostic imaging and cancer treatment. They must receive specialized training in a nuclear pharmacy program and certification as a Board Certified Nuclear Pharmacist. Nuclear pharmacies must be properly designed and operated to safely handle radioactive materials, with adequate shielding, quality control testing, storage, dispensing, and waste disposal procedures to protect personnel.