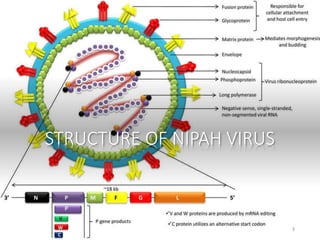
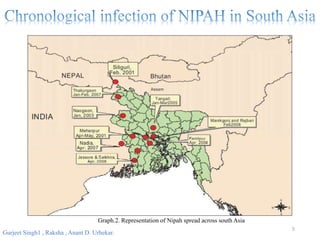
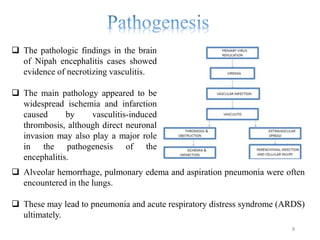
Nipah virus (NiV) is an emerging zoonotic pathogen that causes severe disease in humans and animals, first identified in Malaysia during a 1998-1999 outbreak. Recent outbreaks in Kerala, India, have led to multiple fatalities, with transmission occurring through contaminated date palm sap or close contact with infected individuals. There is currently no specific treatment for NiV, and prevention focuses on supportive care and hygiene measures.