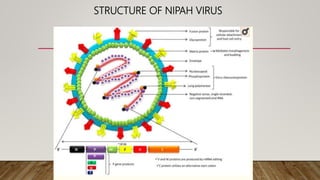
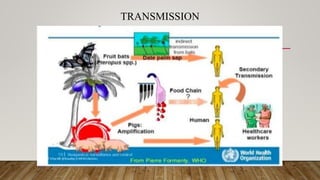
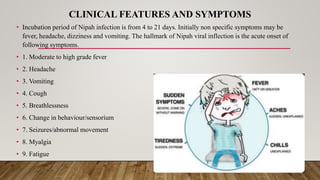
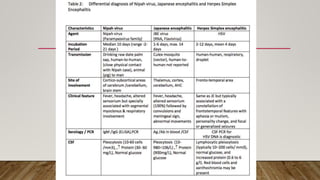
Nipah virus is an emerging zoonotic virus that causes severe disease in humans and animals. It was first identified during an outbreak among pig farmers in Malaysia and Singapore. Since then, outbreaks have occurred regularly in Bangladesh. The virus is transmitted from its natural reservoir of fruit bats to both animals and humans. Symptoms in humans range from asymptomatic infection to acute respiratory infection and fatal encephalitis. There is currently no approved vaccine or antiviral treatment for Nipah virus infection.