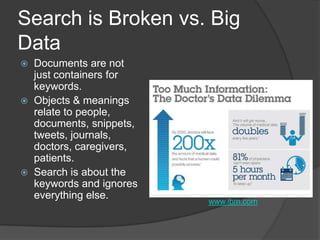
The document outlines the NIH's BD2K initiative, which seeks to invest $100 million to enhance the accessibility, analysis, and sharing of biomedical research data. It highlights the challenges of data sharing among researchers and emphasizes the need for improved infrastructure and analytical tools to support a collaborative research ecosystem. The document also discusses the importance of integrating multiple datasets to better reflect the real-world population in clinical studies and promote open access to research findings.