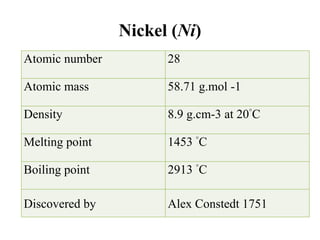
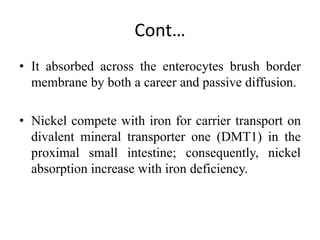
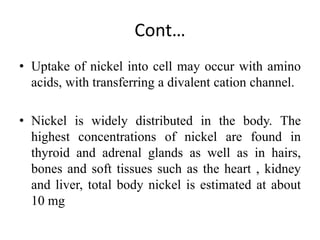
Nickel is a transition metal that is commonly found in the earth's crust. It is used in metal alloys like stainless steel. Nickel is absorbed through the intestine and skin and transported through the blood, where it binds to proteins. The highest concentrations are found in organs like the thyroid, adrenals, bones, heart and kidneys. Most nickel is excreted in the urine, though some is also lost in bile and sweat. Nickel exposure has been linked to lung cancer and dermatitis.