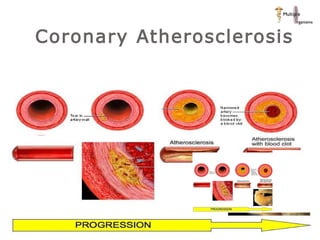
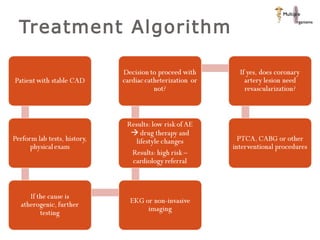
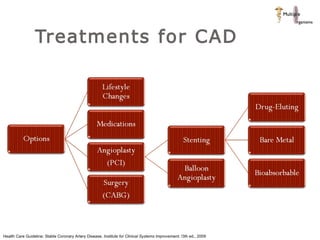
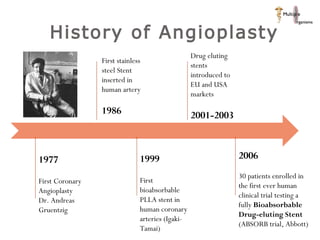
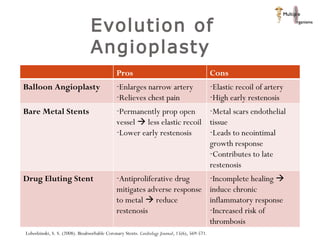
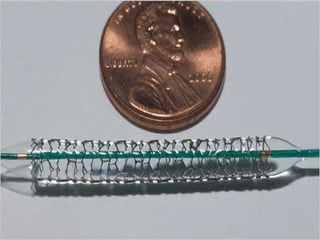
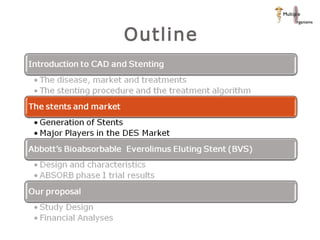
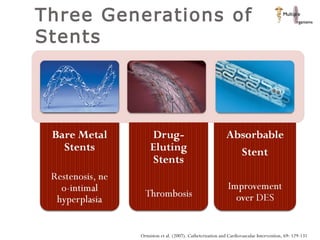
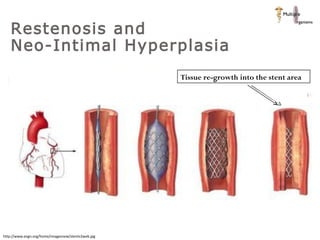
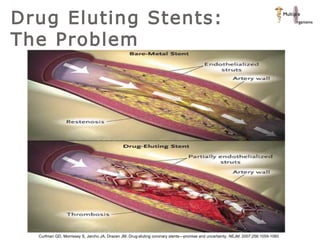
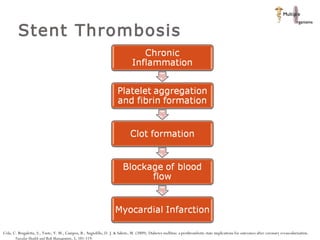
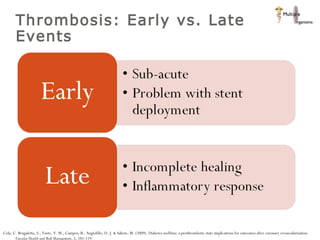
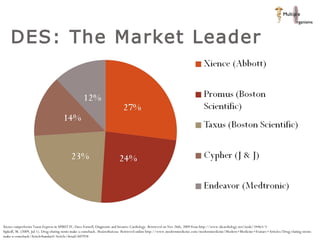
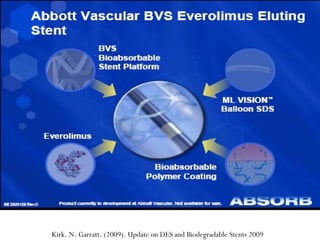
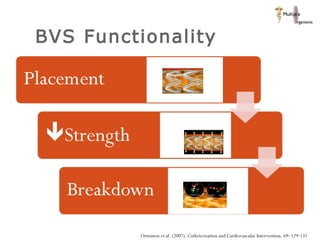
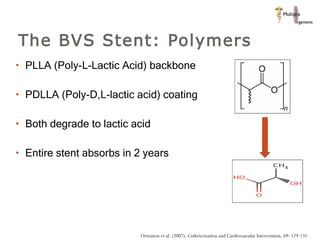
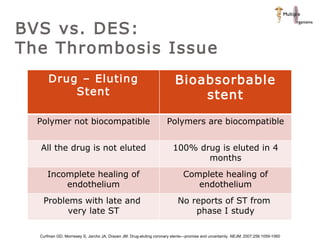
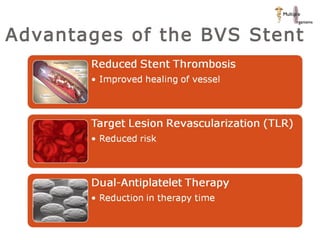
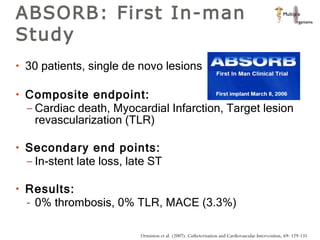
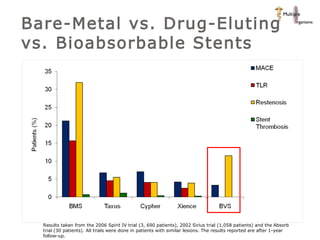
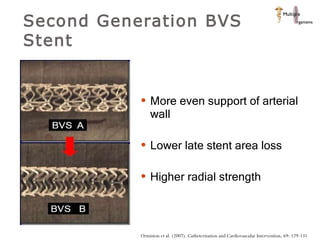
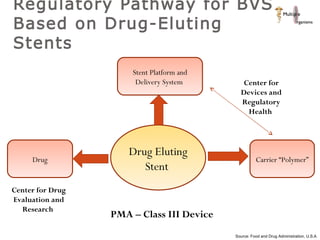
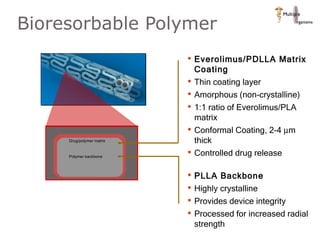
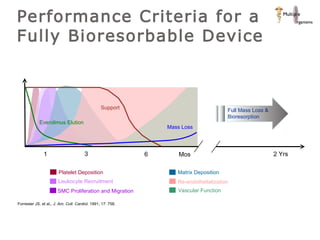
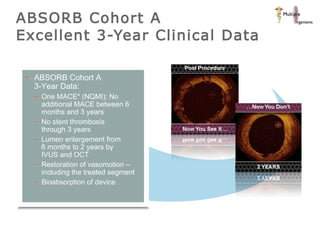
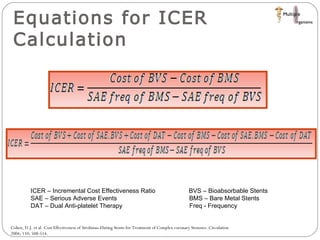
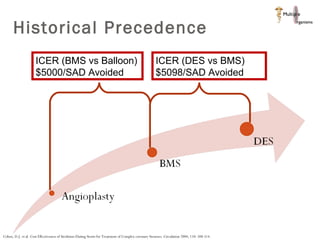
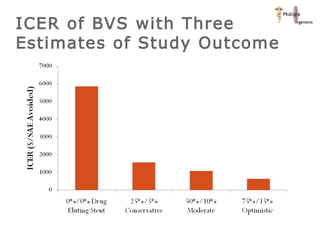
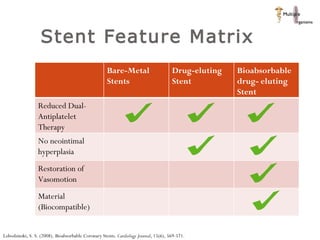
The document discusses newer trends in interventional cardiology, focusing on developments in stent technology, including bare metal stents, drug-eluting stents, and bioabsorbable stents. It describes how stents have evolved from balloon angioplasty to using drugs and biodegradable materials to prevent restenosis. Bioabsorbable stents potentially offer reduced need for long-term blood thinners and restoration of normal vascular function once absorbed. Clinical trials so far show bioabsorbable stents perform similarly to drug-eluting stents with no reported stent thromboses.